2,3-unsaturated galactopyranoside compound and synthesis method thereof
A synthesis method and compound technology are applied in the field of synthesis of 2,3-unsaturated galactosinolate compounds and can solve the problem of low selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
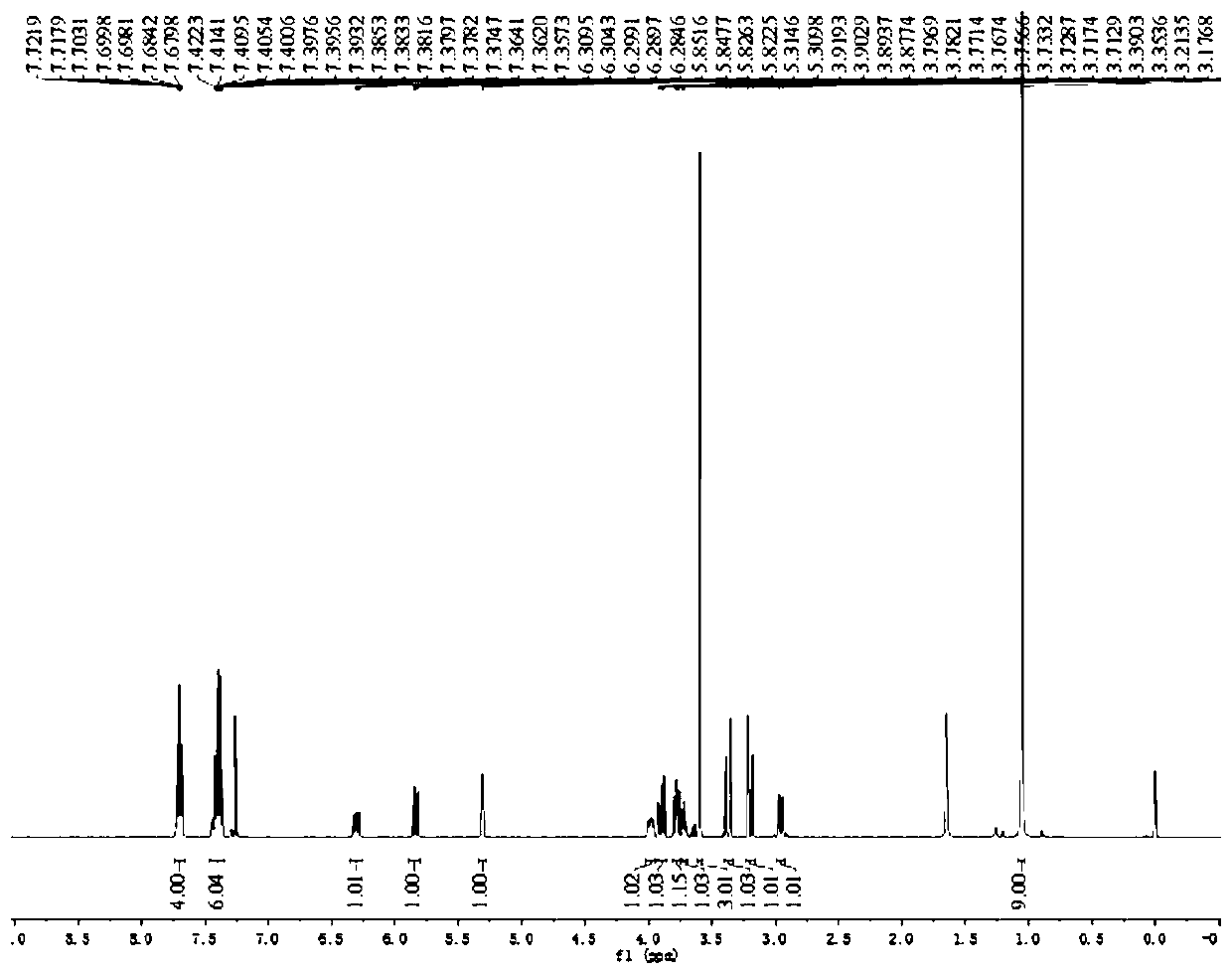
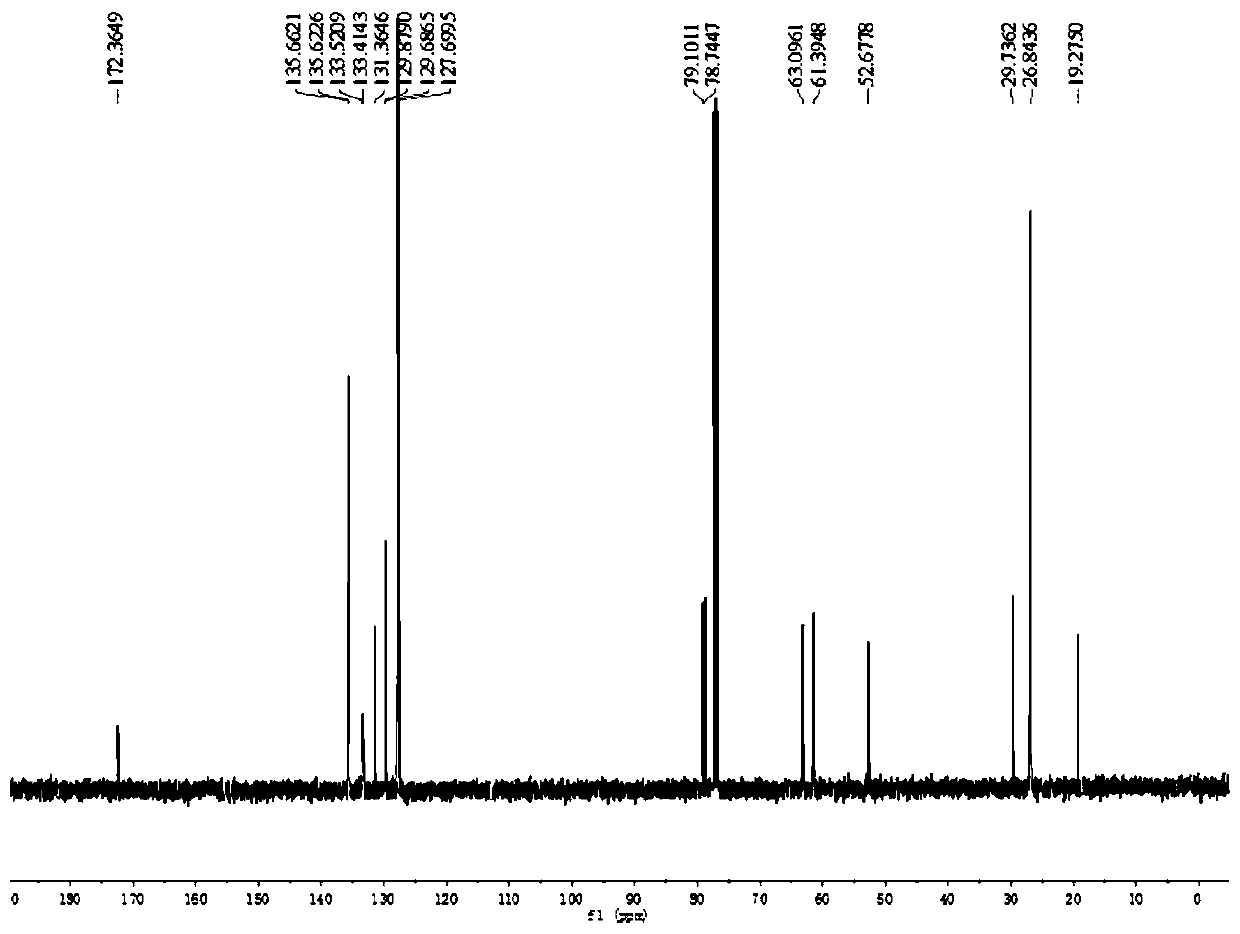
[0027] Taking galactenose as an example, in the optimization experiments using different catalysts and ligands, the yield varies greatly, as follows:
[0028]
[0029]
[0030] All tests adopt 0.1mmol galactenose and 0.2mmol p-cresol, 5mol% Pd catalyst, 20mol% phosphine ligand in 2mL solvent, stirring reaction at room temperature; separation yield; =30:1.
[0031] Reaction conditions screening tests show that no ligand or catalyst reaction can be carried out (entries 1-2), using Pd2(dba)3 catalyst, Xantphos ligand in DMF, DMSO, toluene, acetonitrile, carbon tetrachloride and other solvents are all No reaction (entries 2-7), when DCM was used as solvent, there was 67% of the target reaction product (entry 8). We then proceeded to screen different catalysts (entries 9-13) to find that Pd 2 (dba) 3 It is still the optimal catalyst, and then screened different ligand types and temperatures (entries 14-22), and determined the optimal condition as (entry 20), with Pd2(dba)3...
Embodiment 2
[0035] Embodiment 2: the synthesis of compound 3
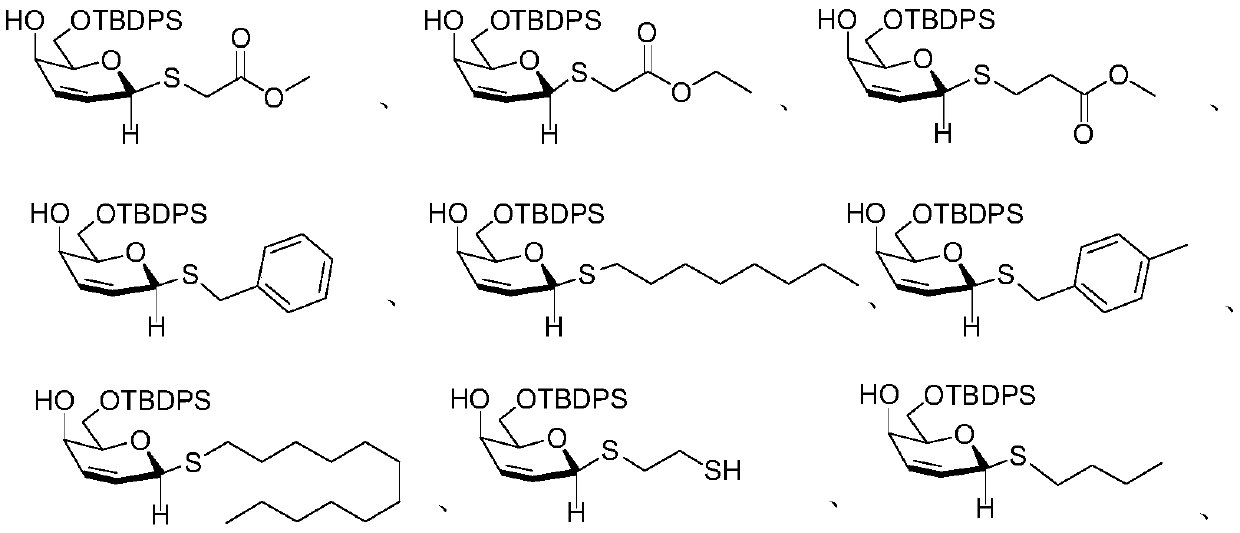
[0036] Tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 2.3mg, 0.0025mmol), 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene (Xantphos, 5.8mg, 0.0010mmol) and 3,4-O-carbonate hemi-emulsion Glucose 1 (0.1 mmol) was added to 2 mL of dichloromethane and methyl thioglycolate (0.2 mmol). Stir at room temperature, TLC detects the reaction process, when the vinyl sugar raw material completely disappears, terminate the reaction, extract and collect the organic phase, distill off the solvent under reduced pressure to obtain the crude product, and then use petroleum ether / ethyl acetate solution as the mobile phase for column chromatography to obtain 4-Hydroxy-2,3-unsaturated glucosinolate 3 (91% yield).
Embodiment 3
[0037]Embodiment 3: the synthesis of compound 4
[0038] Tris(dibenzylideneacetone)dipalladium (Pd 2 (dba) 3 , 2.3mg, 0.0025mmol), 4,5-bis(diphenylphosphine)-9,9-dimethylxanthene (Xantphos, 5.8mg, 0.0010mmol) and 3,4-O-carbonate hemi-emulsion Glycol 1 (0.1 mmol) was added to 2 mL of dichloromethane and ethyl thioglycolate (0.2 mmol). Stir at room temperature, TLC detects the reaction process, when the vinyl sugar raw material completely disappears, terminate the reaction, extract and collect the organic phase, distill off the solvent under reduced pressure to obtain the crude product, and then use petroleum ether / ethyl acetate solution as the mobile phase for column chromatography to obtain 4-Hydroxy-2,3-unsaturated glucosinolate 4 (95% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com