Graphite-phase carbon nitride material for catalytic reduction of p-nitrophenol as well as preparation method and application of graphite-phase carbon nitride material
A technology of graphite phase carbon nitride and p-nitrophenol, which is applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of high catalyst cost and unsuitability for large-scale production, and achieve Conducive to adsorption, multiple active sites, and increased specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
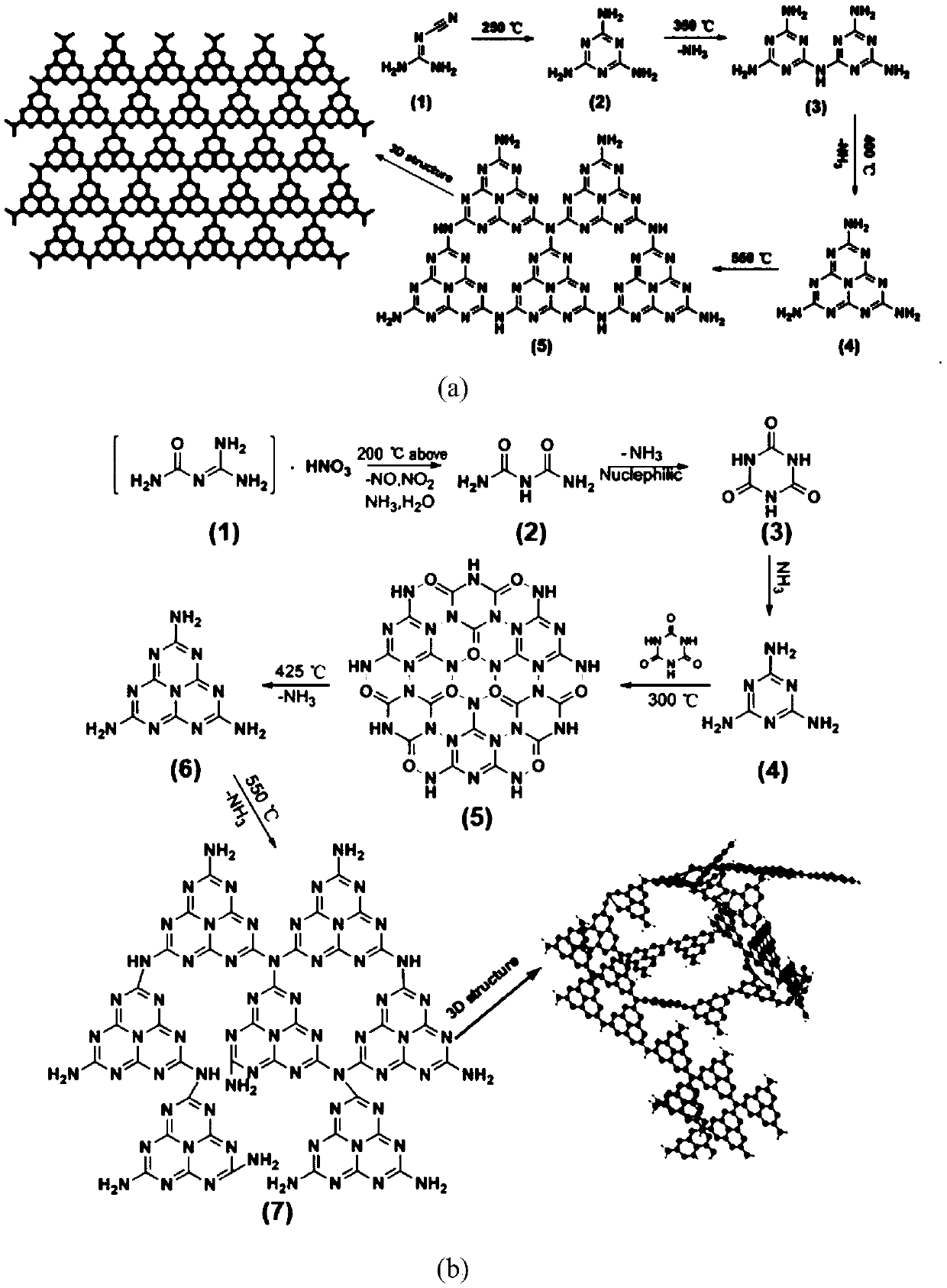
[0021] The preparation steps of graphite phase carbon nitride of the present invention and catalytic reduction process are as follows:
[0022] In the first step, dicyandiamide is dissolved in deionized water, heated and stirred at 40-45 °C for 30-60 min;
[0023] In the second step, slowly drop concentrated nitric acid with a volume ratio of 1:5 to 8 into the solution of the first step, and stir for 120-180 min;
[0024] In the third step, the reaction solution in the second step is cooled and crystallized in a mixed solution of ice and water, dried at 50-60° C., and dried to obtain amidinourea nitrate;
[0025] The fourth step is to roast the amidinourea nitrate obtained in the third step at 550±10° C. for 2-4 h to prepare graphite phase carbon nitride;
[0026] The fifth step, weigh an appropriate amount of graphite phase carbon nitride in the catalytic tube, add p-nitrophenol ( p- NP) and reducing agent sodium borohydride (NaBH 4 ), the reaction was carried out at room ...
Embodiment 1
[0029] In the first step, 2 g of dicyandiamide was dissolved in 40 mL of deionized water, heated and stirred at 40°C for 60 min;
[0030] In the second step, slowly drop concentrated nitric acid (5mL) with a volume ratio of 1:8 into the solution of the first step, and stir for 120 min;
[0031] In the third step, the reaction solution in the second step is cooled and crystallized in a mixed solution of ice and water, dried at 60° C., and dried to obtain amidinourea nitrate;
[0032] In the fourth step, calcining the amidinourea nitrate obtained in the third step at 550±10°C for 4 h to obtain graphitic carbon nitride 5H-CN;
[0033] The fifth step, weigh 1 mg 5H-CN in the catalytic tube, add 25mLH 2 O. 25mL p- NP (20mg / L)
[0034] and 25 mg NaBH 4 , react at room temperature.
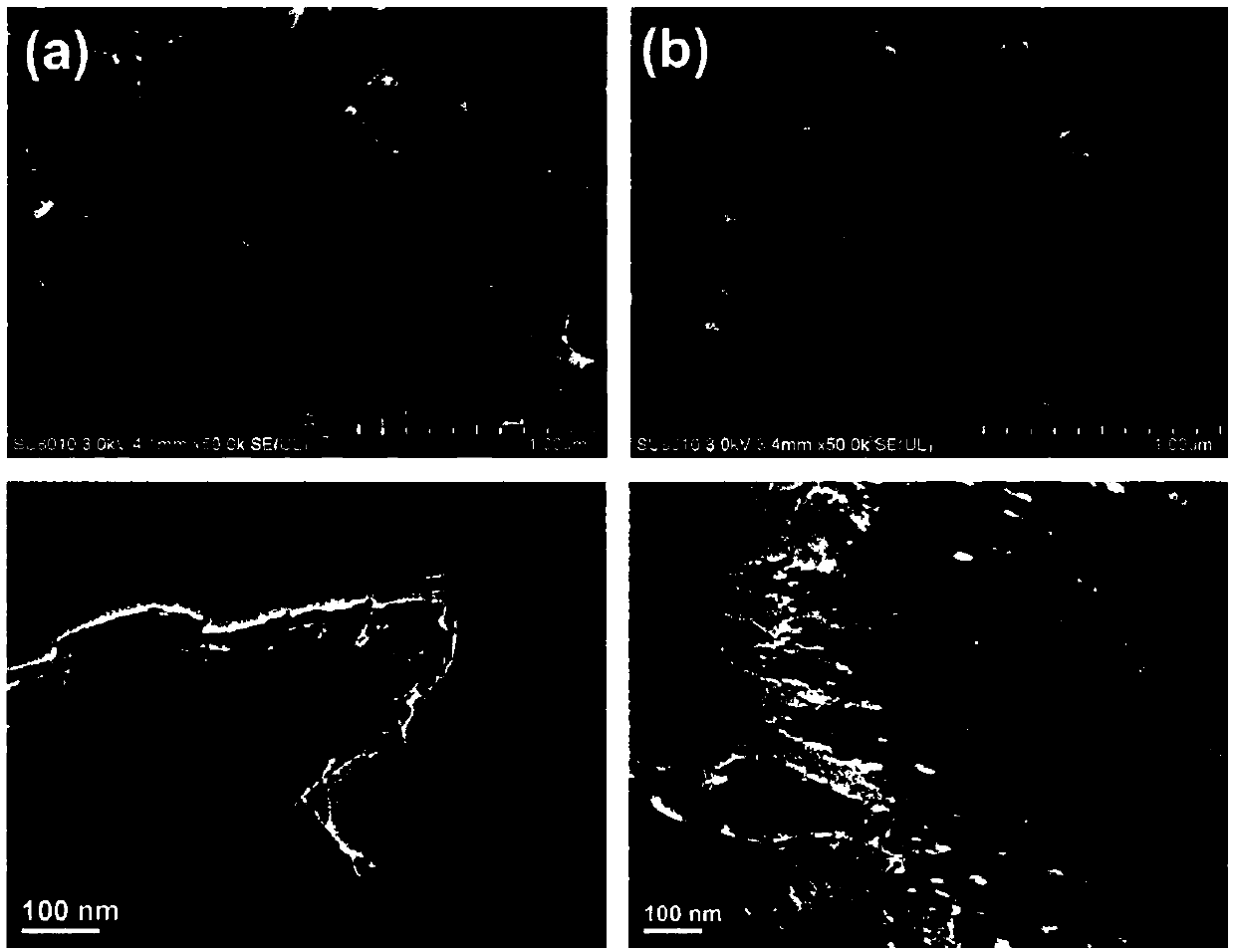
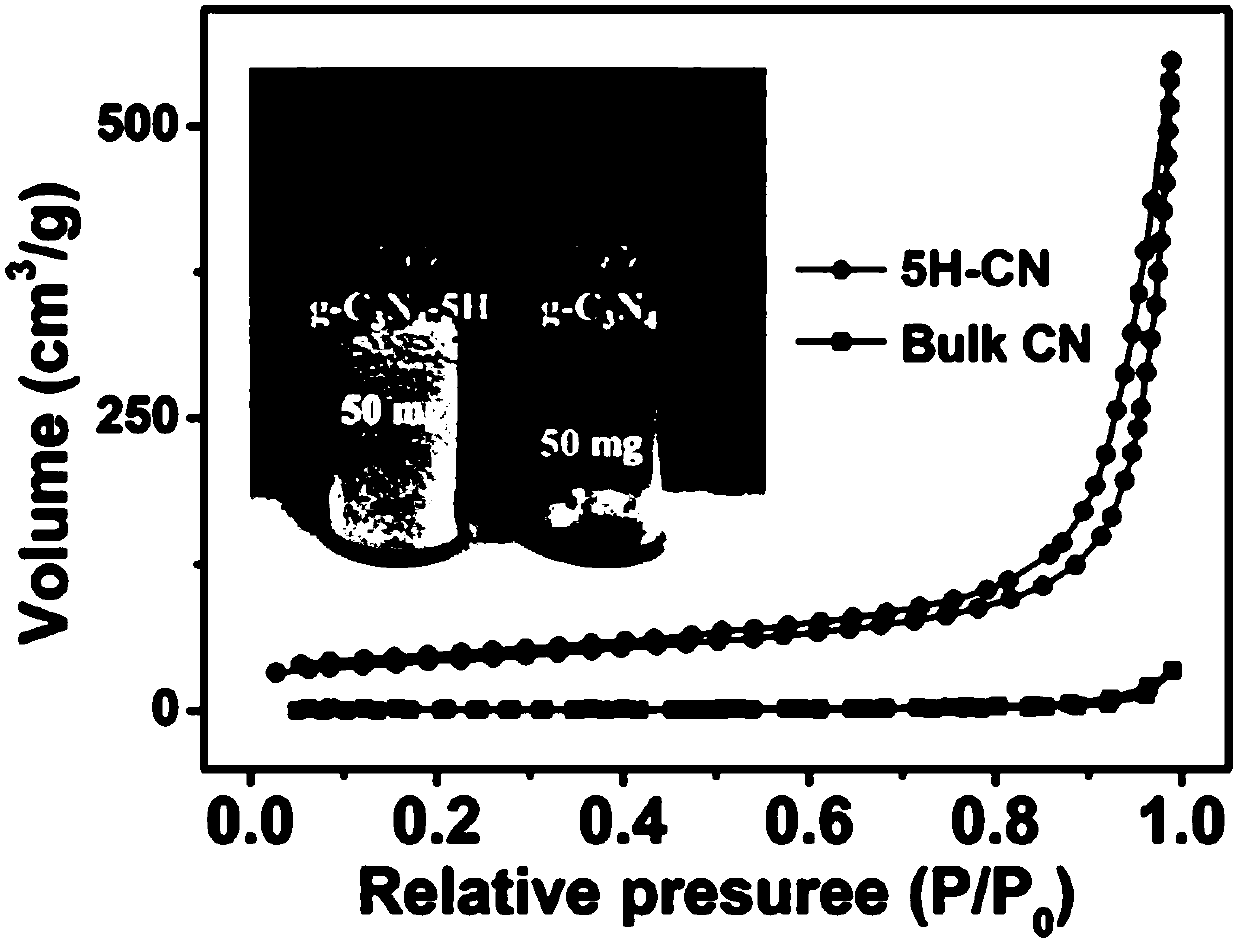
[0035] The prepared 5H-CN, its field emission scanning electron microscope and transmission electron microscope such as figure 2 As shown, BET as image 3 shown.
[0036] Catalytic reduction of ...
Embodiment example 2
[0038] In the first step, 2 g of dicyandiamide was dissolved in 40 mL of deionized water, heated and stirred at 45 °C for 30 min;
[0039] In the second step, slowly drop concentrated nitric acid (8 mL) with a volume ratio of 1:5 into the solution of the first step, and stir for 180 min;
[0040] In the third step, the reaction solution in the second step is cooled and crystallized in the ice-water mixture, and dried at 50°C;
[0041] In the fourth step, the amidinourea nitrate obtained in the third step was calcined at 550 °C for 2 h to prepare graphitic carbon nitride 8H-CN.
[0042] The fifth step, weigh 1 mg8H-CN in the catalytic tube, add 25mLH 2 O. 25mL p- NP (20mg / L)
[0043] and 25 mg NaBH 4 , react at room temperature.
[0044] The result is as Figure 4 Shown, room temperature catalytic reduction p- After 14 min of reaction, the reduction rate of NP reached 90.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com