A kind of method for synthesizing imidacloprid in high yield
A technology for imidacloprid and compounds, applied in the field of imidacloprid synthesis and imidacloprid synthesis, can solve the problems of poor safety, poor stability, easy to produce explosions, etc., and achieve the effects of easy industrialization, good product purity, and high process safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
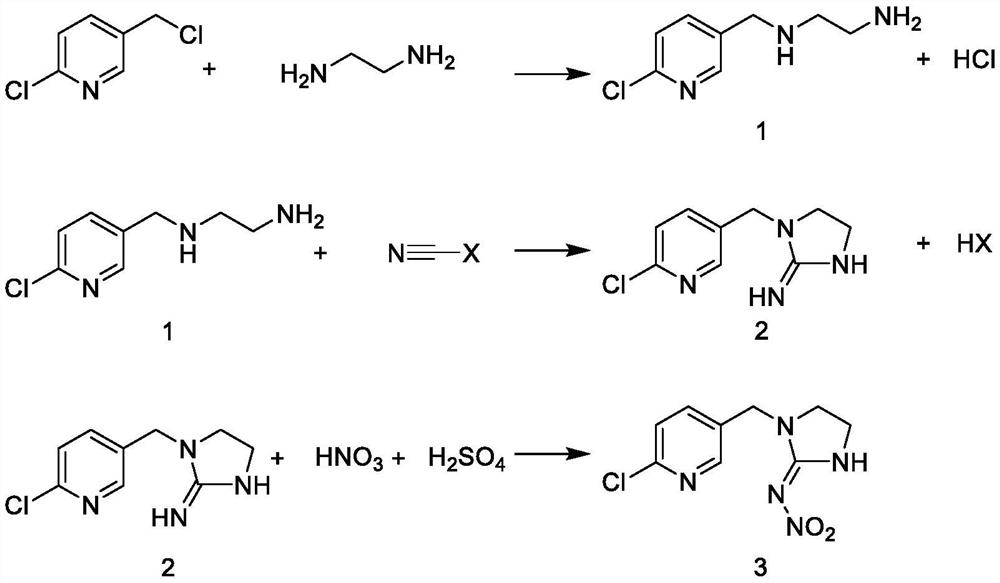
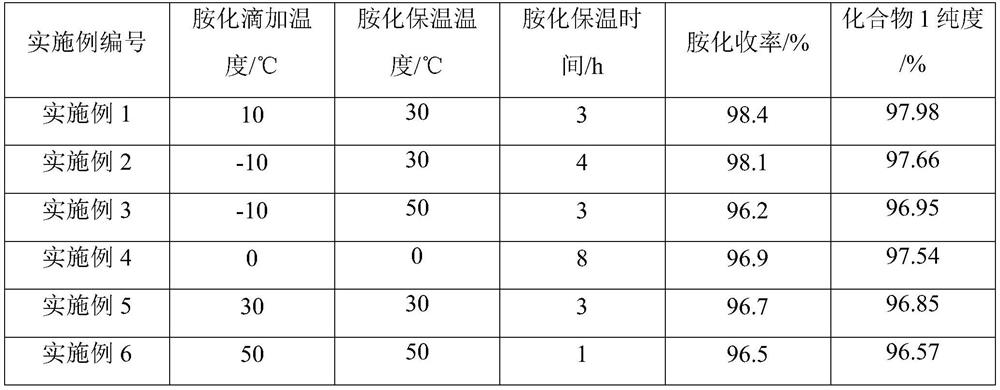
[0019] Amination: 30.0g of 50% NaOH, 112.0g of ethylenediamine and 200mL of acetonitrile were put into a 1000mL four-necked bottle; 63.0g of 95.88% 2-chloro-5-chloromethylpyridine was dissolved in 100mL of acetonitrile and placed in a 250mL dropping funnel inside. A 1000mL four-neck flask was placed in an ice-water bath, the temperature of the system was controlled at 8-10°C, and the dropping time was controlled at about 1h. After the dropwise addition of the acetonitrile solution of 2-chloro-5-chloromethylpyridine, the temperature of the system began to rise. After the system was heated to 30° C., it was kept for 3 hours, and the reaction process ended. After the reaction is over, the solvent is recovered by precipitation, the temperature is lowered, suction filtration, and the residue obtained by negative pressure precipitation is N 1 -((6-Chloropyridin-3-yl)methyl)ethane-1,2-diamine imine 69.51 g, product purity 97.98%, yield 98.4%.
[0020] Cyclization: Put 400 g of an a...
Embodiment 2-6
[0023] On the basis of embodiment 1, change amination reaction temperature, time, other conditions are constant, and reaction result is as follows:
[0024]
Embodiment 7-9
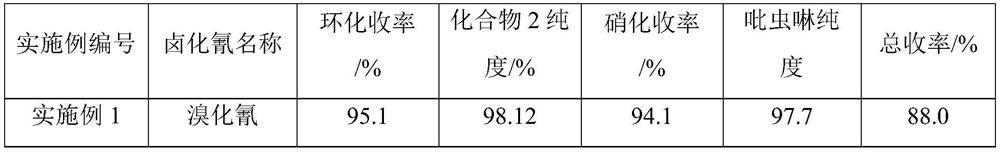
[0026] On the basis of Example 1, using compound 1 obtained by amination as a raw material, the cyclization raw material cyanogen bromide is changed into other cyanogen halides, and other conditions remain unchanged, the results are as follows:
[0027]
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com