Method for deep oxidation treatment on organic wastewater by activating peroxymonosulfate based on visible light-assisted complexing iron ions
A monoperoxyhydrogensulfate and organic wastewater technology, which is applied in oxidized water/sewage treatment, chemical instruments and methods, light water/sewage treatment, etc., can solve the problem of low CODcr removal efficiency and inability to continuously activate PMS oxidation in organic wastewater Free radicals and other problems, to achieve good CODcr removal effect, reduce treatment costs, and strong oxidation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
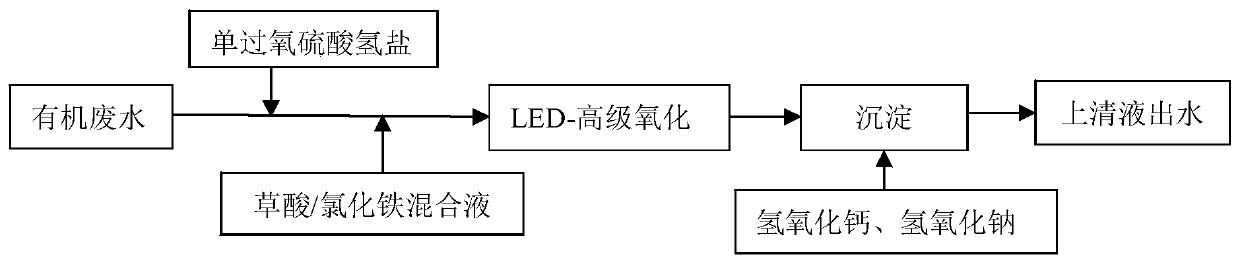
[0033] Under normal temperature conditions, take 250mL of aniline solution with CODcr of 200mg / L and pH=5 in 6 beakers and mark them as 1#, 2#, 3#, 4#, 5#, 6# respectively.
[0034] (1) Add PMS solution to the 1# beaker and mix evenly, turn on the LED light source to irradiate the solution in the beaker at the same time, start timing the reaction, add appropriate amount of lye and calcium hydroxide after 2 hours of reaction and let it stand for 1 hour;
[0035] (2) In the 2# beaker, first add PMS solution and mix evenly, then add ferric chloride solution, the reaction starts timing, after 2 hours of reaction, add appropriate amount of lye and calcium hydroxide and let it stand for 1 hour;
[0036] (3) First add PMS to the 3# beaker and mix evenly, then add freshly prepared ferrous sulfate solution, the reaction starts timing, after reacting for 2 hours, add appropriate amount of lye and calcium hydroxide and let it stand for 1 hour;
[0037](4) First add PMS to the 4# beaker a...
Embodiment 2
[0045] Under normal temperature conditions, take 250mL of aniline solution with CODcr of 200mg / L and pH=10 in 3 beakers and mark them as 1#, 2#, 3#, 4# respectively.
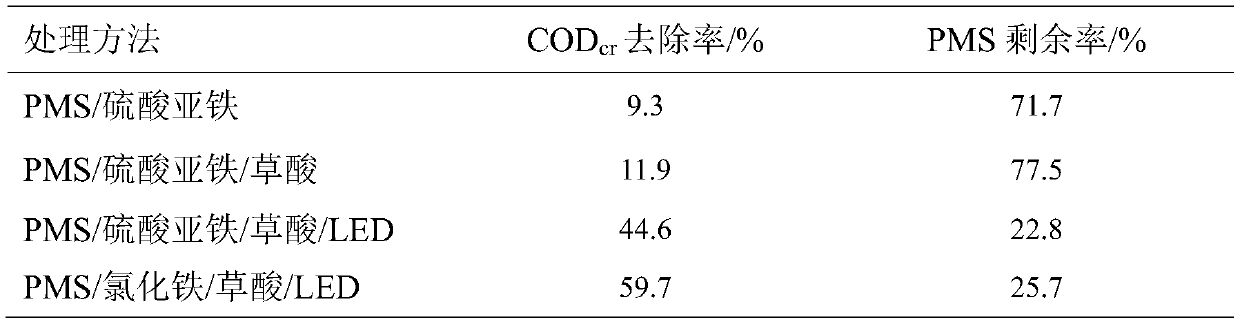
[0046] (1) First add PMS to the 1# beaker and mix evenly, then add freshly prepared ferrous sulfate solution, start timing the reaction, add lye and calcium hydroxide after 2 hours of reaction and let it stand for 1 hour;
[0047] (2) First add PMS to the 2# beaker and mix evenly, then add freshly prepared mixed solution of oxalic acid and ferrous sulfate, start timing the reaction, add lye and calcium hydroxide after 2 hours of reaction and let it stand for 1 hour;
[0048] (3) First add PMS to the 3# beaker and mix well, then add the freshly prepared mixed solution of oxalic acid and ferrous sulfate, at the same time turn on the LED light source to illuminate the solution in the beaker, the reaction starts timing, and add lye and hydrogen after 2 hours of reaction Calcium oxide was allowed to stand for 1 hour;...
Embodiment 3
[0055] Under normal temperature conditions, take 250mL of aniline solution with CODcr of 200mg / L and pH=7 in 2 beakers and mark them as 1# and 2# respectively.
[0056] The PMS in this embodiment adopts KHSO 5 .
[0057] (1) First add PMS to the 1# beaker and mix well, then add the freshly prepared mixed solution of oxalic acid and ferric chloride, at the same time turn on the LED light source to illuminate the solution in the beaker, the reaction starts timing, after 2 hours of reaction, add lye and let it stand 1 hour;
[0058] (2) First add PMS to the 2# beaker and mix well, then add the freshly prepared mixed solution of oxalic acid and ferric chloride, and at the same time turn on the LED light source to illuminate the solution in the beaker, the reaction starts timing, and add lye and hydrogen after 2 hours of reaction Calcium oxide was allowed to stand for 1 hour;
[0059] Control the volume of 1#-2# beakers to be 500mL after adding all the solutions, and the COD con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com