Application of dimethyl itaconate in prevention and treatment of ulcerative colitis and canceration of ulcerative colitis
A technology for dimethyl itaconate and ulcerative colitis is applied in the application field of dimethyl itaconate in the prevention and treatment of ulcerative colitis and its carcinogenesis, so as to reduce the risk of colon cancer and prevent ulcerative colitis. effects of colitis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] DI effectively inhibits DSS-induced ulcerative colitis
[0044] 1. The test method is as follows:
[0045] (1) The 8-week-old mice were randomly divided into two groups, the ears of the mice were punched and marked, and the body weight of the mice was recorded once a day.
[0046] (2) After the mice were observed and stabilized in the animal room for one week, they drank water with 3% DSS solution for 5 days, and then drank normal water for 5 days.
[0047] (3) While administering 3% DSS solution in drinking water, one group was injected with 200 μL PBS (containing 20 mg DI), and the other group was injected with 200 μL PBS, and no administration was given during normal drinking water.
[0048] (4) After 10 days, observe the stool properties or blood in the stool, and terminate the experiment.
[0049] (5) After the mice were killed by cervical dislocation, the colorectum was separated and removed, and pathological staining, QPCR detection, and Western blotting were per...
Embodiment 2DI
[0058] Example 2 DI can reduce the risk of developing colon cancer from ulcerative colorectum
[0059] 1. The test method is as follows:
[0060] (1) The mice were randomly divided into two groups, the ears of the mice were punched and marked, and the body weight of the mice was recorded every five days.
[0061] (2) After the mice were observed and stabilized in the animal room for one week, they were intraperitoneally injected with a dose of 10 mg / kg.
[0062] (3) After 5 days of injection, the mice were continuously given 2% DSS solution drinking water for 5 days, and then given normal drinking water for 16 days, a total of 3 cycles.
[0063] (4) After the three cycles, observe the stool properties or blood in the stool, and observe whether there is tumor prolapse in the anus of the mouse. If there is an obvious tumor in the anus of the mouse or the test period reaches 80 days, the experiment can be terminated.
[0064] (5) After the mice were successfully anesthetized, t...
Embodiment 3
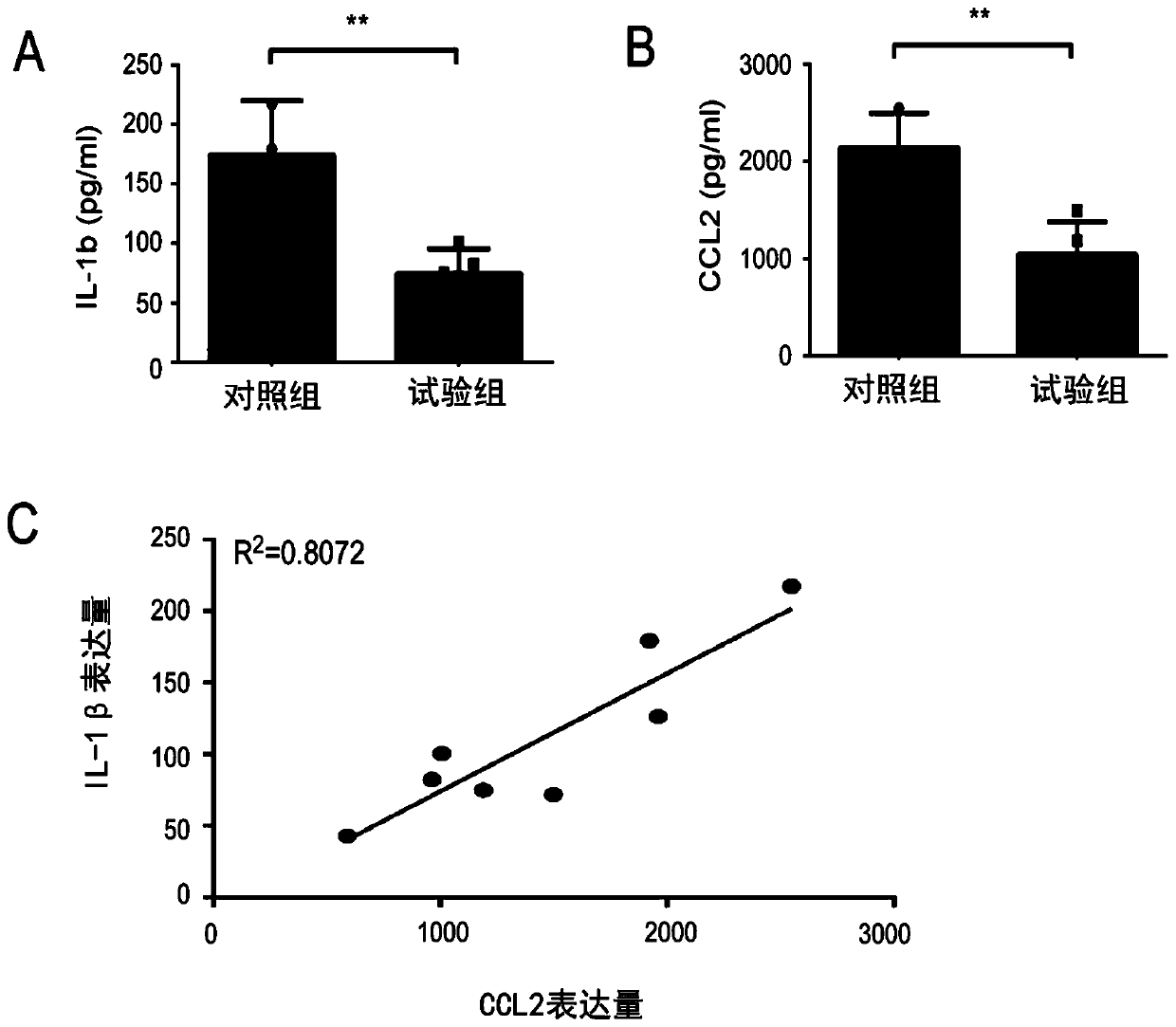
[0072] Example 3 DI can reduce the secretion of IL-1β and CCL2
[0073] 1. The test method is as follows:
[0074] (1) Before the experiment, put the detection kit at room temperature to balance for 20 minutes, and all liquid components should be shaken well before use. Frozen serum was thawed on ice.
[0075] (2) Take out the required strips from the aluminum foil bag, and seal the remaining strips in a ziplock bag and store them at 4°C until use.
[0076] (3) Set up 6 protein standard products, the standard product is 2000pg / ml, and the concentrations of protein standard products are sequentially diluted to 500pg / ml, 250pg / ml, 125pg / ml, 62.5pg / ml, 31.25pg / ml, 0pg / ml ml, each concentration of 100 μl sample was added to the well plate.
[0077] (4) After 5-fold dilution of the sample, 100 μl was added to the well plate, covered with a plate sticker, and incubated at 37°C for 2 hours.
[0078] (5) Discard the liquid, shake dry, no need to wash.
[0079] (6) After diluting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com