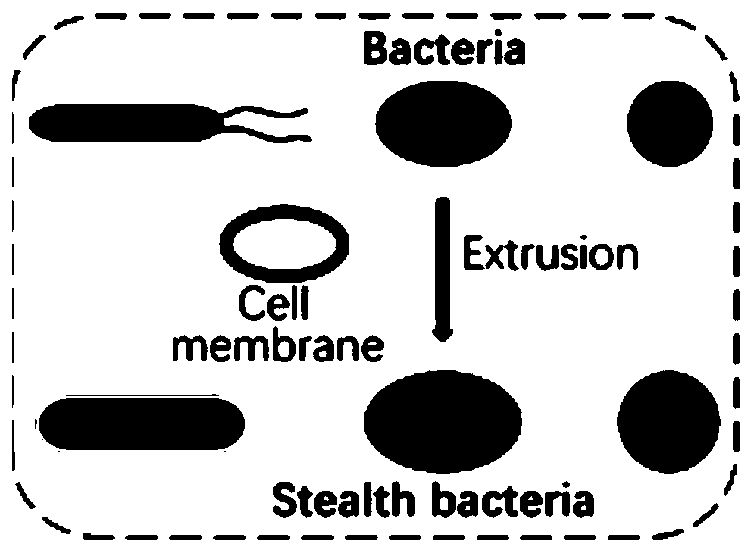
Surface-modified microorganism and preparation method and application thereof
A surface modification and microbial technology, applied in the biological field, can solve the problems of unfavorable expansion of production, inability to improve the effect of bacterial prevention and treatment, and short residence time, so as to improve survival rate and tolerance, increase colonization and enrichment ability, The effect of reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0105] The experimental methods used in the examples are conventional research methods in the fields of biochemistry, molecular biology or medical detection. Wherein, Escherichia coli Nissle 1917 (hereinafter referred to as EcN), Enterococcus faecalis (E.faecalis), Staphylococcus aureus (S.aureus) and Salmonella typhimurium (Salmonella typhimurium) used in the present invention were purchased from China General Microorganism Culture Collection center.
[0106] Cell lines (Hela, CT26 and 4T1) were from the American Species Culture Collection (ATCC).
[0107] Plasmids pBBR1MCS2-Tac-mCherry, pBBR1MCS2-Tac-GFP and pMD18-luxCDABE were purchased from domestic suppliers.
Embodiment 1
[0110] EcN (E. coli Nissle) carrying pBBR1MCS2-Tac-mCherry, pBBR1MCS2-Tac-GFP or pMD18-luxCDABE were grown overnight at 37°C in LB medium containing antibiotics. E. faecalis, S. aureus and S. typhimurium were grown in tryptic soy broth (TSB) medium at 37°C. The overnight culture was diluted 1:50 into fresh LB medium and grown at 37 °C for 3 h. Bacteria were harvested by centrifugation at 4200 rpm for 10 minutes and resuspended in ice-cold PBS. Bacterial counts were determined by diluting the bacterial suspension, growing overnight at 37°C on LB agar plates and counting colony forming units (CFU).
Embodiment 2
[0112] Red blood cell membranes were prepared according to the method reported in reference 1. Briefly, whole blood was extracted through the eye socket from anesthetized male ICR mice (6–8 weeks) purchased from Jiesijie Experimental Animal Center (Shanghai, China), and then centrifuged at 800 g for 5 min at 4 °C to Remove serum and buffy coat. After three washes in cold 1 × PBS, the obtained packaged RBCs were suspended in 0.25 × PBS on ice for 30 min and then centrifuged at 10000 rpm for 5 min to remove hemoglobin. The pink pellet was washed with cold 1x PBS and stored at -20 °C for further use. The resulting cell membranes are shown in Figure 2B, where Figure 2A shows (untreated) erythrocytes.
[0113] All animal procedures complied with the Shanghai Medical Laboratory Animal Care Guidelines. The animal protocol was approved by the Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com