Monoclonal antibody targeting nerve growth factor and application thereof
A nerve growth factor and monoclonal antibody technology, applied in the field of biochemistry, can solve problems such as hallucinations, drug abuse, abuse and addiction, and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Production and screening of murine antibodies
[0033] BALB / c mice were immunized multiple times with His-tagged NGF, and mice with high serum titers were obtained. The splenocytes of the mouse with the highest titer are fused with SP20 myeloma cells to form hybridoma cells, and then screened for binding. The screening method is "direct ELISA", that is, the human antigen (extracellular region) is coated on the ELISA plate , incubated with the culture supernatant, and then chromogenically screened using biotin-labeled goat anti-mouse Fc antibody, Strepavidin-HRP, and TMB substrate. Through combination screening, a large number of binding clones were obtained. Finally, the two antibodies with the strongest affinity were selected and named: 1D4 and 5F2.
Embodiment 2
[0034] Example 2: Sequence analysis of murine antibodies
[0035] The light and heavy chain cDNAs of 1D4 and 5F2 were cloned and sequenced. The sequences were analyzed using tools on AbYsis combined with manual experience, and the framework regions, complementarity determining regions (CDRs) and invariable regions were annotated. A Genebank protein sequence homology search was performed on the variable regions of 2D3 (by comparing the framework regions and CDR regions), and the results showed that the framework regions were highly homologous to those of various mouse antibodies. No protein sequences homologous to the CDR regions of 1D4 and 5F2 were found. Therefore, 1D4 and 5F2 are completely new murine antibodies whose sequences have not been reported yet.
[0036] The sequencing results of the light and heavy chain variable regions of 1D4 and 5F2 are as follows:
[0037] CDR-H1 (SEQ ID NO: 1 or SEQ ID NO: 7);
[0038] CDR-H2 (SEQ ID NO: 2 or SEQ ID NO: 8);
[0039] CDR-...
Embodiment 3
[0048] Example 3: Antigen affinity analysis
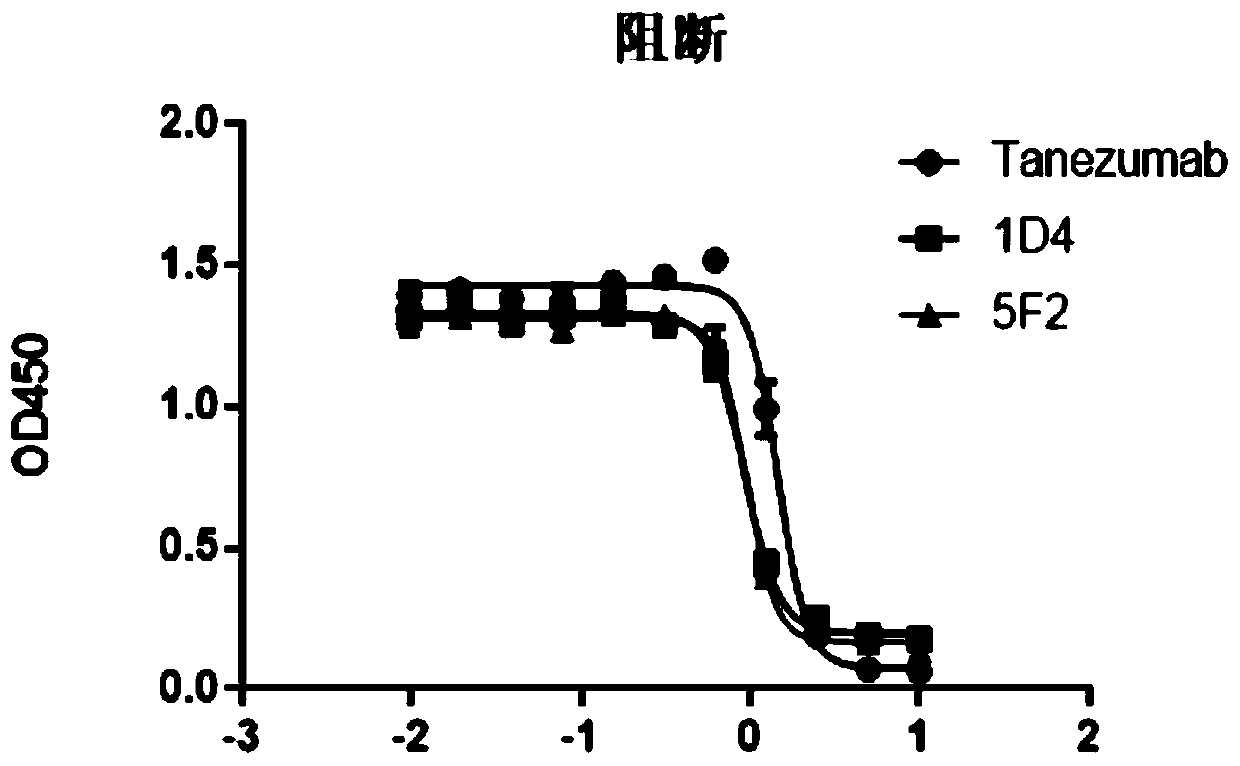
[0049] Coat 1ug / ml antibody protein on the ELISA plate overnight at 4°C, block with blocking solution at room temperature the next day to avoid non-specific binding, then add biotinylated antigen to be detected and incubate at room temperature, add horseradish peroxide Enzyme-labeled streptavidin (Streptavidin-HRP) was used as a chromogenic substrate, and TMB was used to develop color, and the absorbance was measured at 450nm. The corresponding binding curves were made on Prism. The results showed that the affinities of 1D4 and 5F2 to the antigen were 5.2 and 2.9 nM (see figure 1 ), which has a similar affinity to the NGF antibody molecule Tanezumab of Pfizer and Eli Lilly and Company of the United States.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com