Neutralizing antibody against novel coronavirus sars-cov-2 and its application
A sars-cov-2 and coronavirus technology, applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve problems such as affinity differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Screening of fully human antibodies targeting SARS-Cov-2-RBD
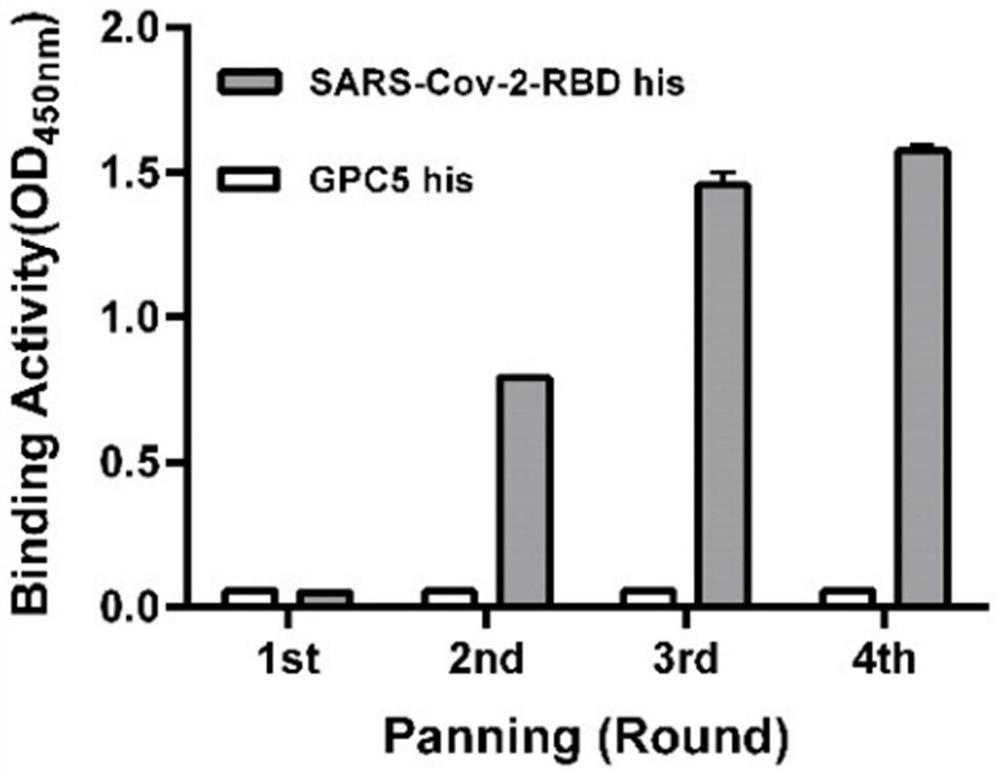
[0038] Using phage display technology, with SARS-Cov-2-RBD-his protein as positive antigen, SARS-Cov-1-RBD-hFc as negative antigen, in Tomlinson I&J phage library (Genservice Ltd., Cambridge, UK, library size 1.47x10 8 ) for differential screening.
[0039] Coat immunoplates with 50 μg / ml SARS-Cov-2-RBD his antigen and SARS-Cov-1-RBD hFc antigen at 4°C overnight; block with PBS solution containing 5% skimmed milk powder and 0.1% Tween-20 at room temperature Immunoplate for 1 hour; phage library at 10 12 Mix pfu with 10% skimmed milk powder PBS solution 1:1, incubate at room temperature for 2 hours, add the blocked SARS-Cov-1-RBD hFc antigen immunoplate (100 μl / well), incubate at room temperature for 1 hour, and perform negative antigen pre-adsorption ; After pre-adsorption, the supernatant was transferred to the blocked SARS-Cov-2-RBD his antigen immunoplate (100 μl / well), and incubated at room...
Embodiment 2
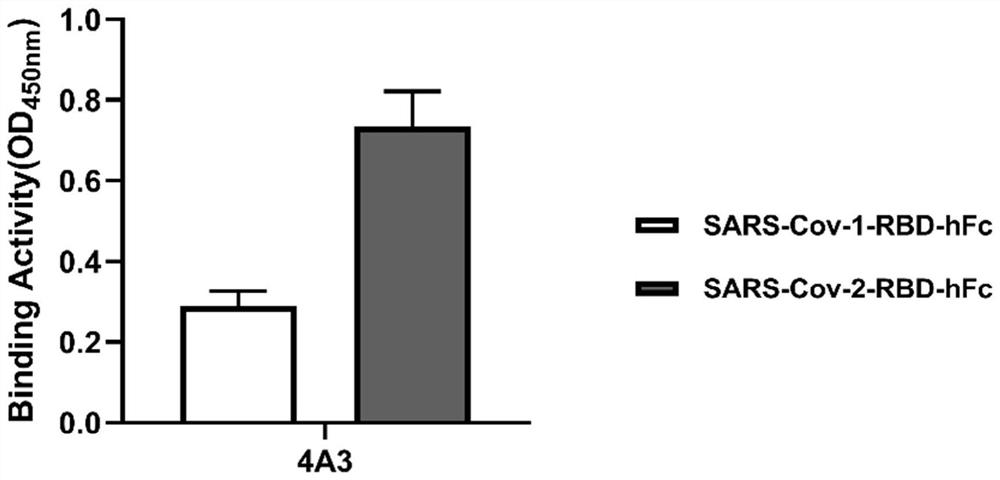
[0050] Example 2, Antigen-specific analysis of antibodies
[0051] In this embodiment, ELISA is used to detect the combination of the 4A3 phage of Example 1 and the SARS-Cov-2-RBD protein.
[0052] The specific process is: use 5 μg / ml of SARS-Cov-1-RBD-hFc and SARS-Cov-2-RBD-hFc to coat the immune plate overnight at 4°C; use 3% skimmed milk powder, 0.05% Tween-20 The PBS solution of 0.05% Tween-20 was used to block the immune plate for 1 hour at room temperature; the 4A3 phage was added to the blocked immune plate (50 μl / well), and incubated for 1 hour at room temperature; the immune plate was washed 3 times with 0.05% Tween-20 PBS solution (340ul / well ); mix HRP / Anti-M13 Monoclonal conjugate with a PBS solution containing 5% skimmed milk powder and 0.05% Tween-20 at a ratio of 1:4000, add to the washed immunoplate (50 μl / well), and incubate at room temperature for 1 hour; Wash the immunoplate 5 times with 0.05% Tween-20 in PBS solution; add TMB chromogenic solution to the im...
Embodiment 3
[0054] Example 3, Expression and Purification of Antibodies
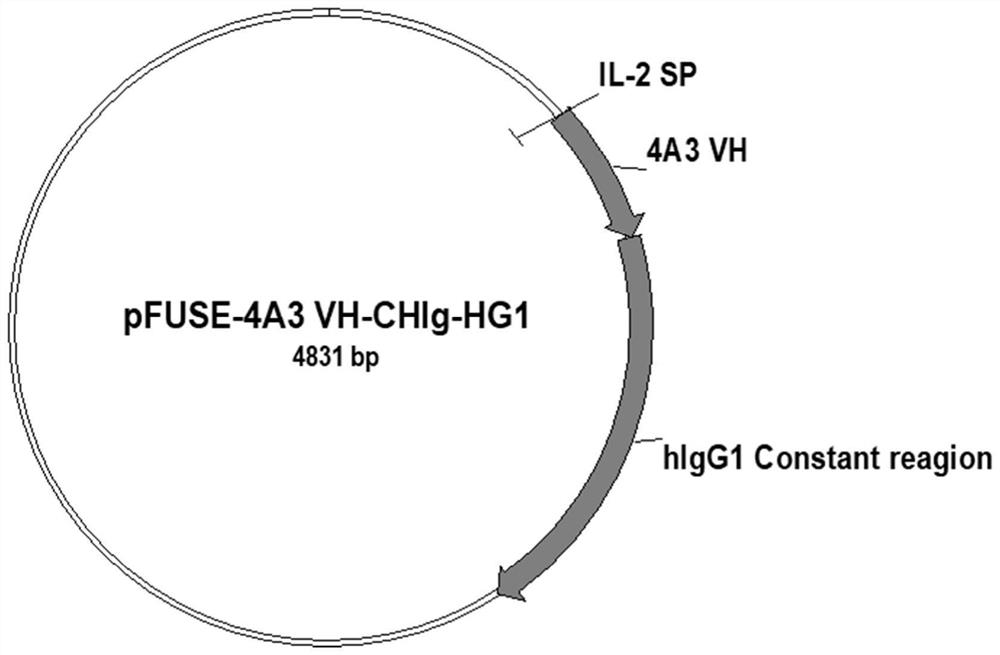
[0055] The heavy chain variable region sequence and the light chain variable region sequence of the 4A3 scFv sequence were inserted into pFUSE-CHIg-HG1 and pFUSE2-CLIg-hk vectors (Invivogen, San Diego, CA), respectively, to construct human IgG1 expression plasmids, such as image 3 , Figure 4 shown. After co-transfection of the above plasmids in 293T cells, the supernatant was collected and purified with a protein A-Agarose separation column, and the purity of the antibody was detected by SDS-PAGE, as shown in Figure 5 shown.
[0056] The specific process is as follows: the heavy chain variable region sequence and the light chain variable region sequence of the 4A3 scFv sequence were inserted into pFUSE-CHIg-HG1 and pFUSE2-CLIg-hk (Invivogen, San Diego, CA), respectively, to construct an adult IgG expression plasmid. 5 million HEK293T cells were planted in a cell culture dish with DMEM medium supplemented with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com