A cisplatin-like drug side effect inhibition or therapeutic target
A side effect and drug technology, applied in the field of cisplatin-based drug side effect inhibition or treatment target, can solve the problems such as large side effects and difficult to inhibit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] LncRNA-MEG3 targets miR-126 to regulate the effect of PI3K / AKT / mTOR signaling pathway on autophagy
[0056] 1. Expression of MEG3 in cisplatin nephrotoxicity
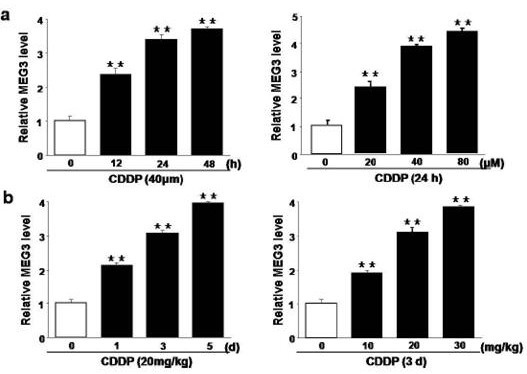
[0057] HK-2 and TCMK-1 renal tubular epithelial cells were selected, treated with cisplatin (20, 40, 60 μM) for 24 h or 40 μM cisplatin for 12, 24 and 48 h, and the expression of MEG3 in the cells was detected by RT-PCR. Expression; C57BL / 6 normal / lung cancer mice, after 3 days of cisplatin (10, 20, 30 mg / kg) or 20 mg / kg cisplatin for 1, 3, 5 days, RT-PCR detection of mouse kidney tissue Expression of MEG3 in . see attached results figure 1 .
[0058] 2. MEG3 inhibits autophagy in renal tubular epithelial cells
[0059] 2.1 In the gene knockout or gene silencing system, detect the effect of MEG3 deletion on autophagy
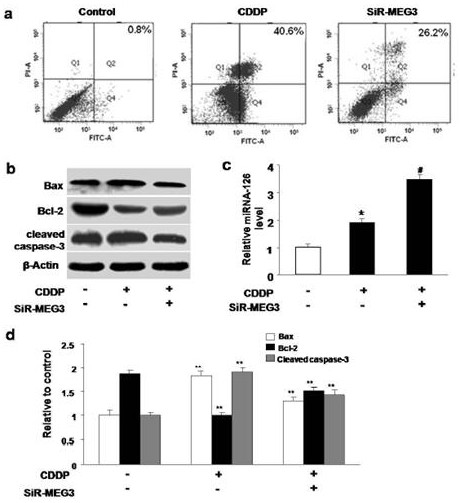
[0060] 1) siRNA or shRNA transfection of HK-2 cell line to silence MEG3: HK-2 cells with good growth status were selected and randomly divided into normal control group, siRNA or shRNA MEG3 gr...
Embodiment 2
[0086] In vitro experiment of paeonol regulating autophagy mediated by LncRNA-MEG3 / miR-126 / mTOR signaling pathway on cisplatin nephrotoxicity
[0087] 1. Establishment and grouping of cisplatin nephrotoxicity cell model
[0088] Renal tubular epithelial cells HK-2 and TCMK-1 cell lines were treated with 40 μM cisplatin for 24 h and divided into three groups: negative control group, MEG3 silencing group and paeonol (40 μM) intervention group.
[0089] 2. The protective effect of paeonol on renal tubular epithelial cell injury ( Figure 5-Figure 7 )
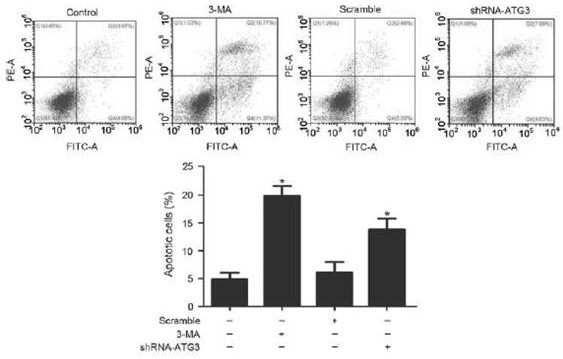
[0090] MTT and CCK-8 were used to detect cell proliferation; flow cytometry and TUNEL were used to detect cell apoptosis; Western blot and immunofluorescence were used to detect the expression of apoptosis-related proteins (P53, Bax, Bcl-2 and caspase-3); ELISA detection Changes in inflammatory factors (IL-2, IL-6, TNF-α, IFN-γ). Rapamycin was added to induce autophagy or 3MA to block autophagy to observe the protective effect o...
Embodiment 3
[0096] Paeonol regulates autophagy mediated by LncRNA-MEG3 / miR-126 / mTOR signaling pathway on the mechanism of cisplatin nephrotoxicity in vivo
[0097] 1. Establishment of an animal model of cisplatin nephrotoxicity
[0098] Lewis lung cancer cells were cultured, tumor cells in the logarithmic growth phase were collected, the concentration of the cell suspension was adjusted to 1×107 / mL, and 0.2 mL of the cell suspension was subcutaneously injected into the right axilla of C57BL / 6 mice to establish Lewis lung cancer mice subcutaneously. Xenograft tumor animal model. On the 8th day after inoculation (the tumor volume is about 8mm), the normal mice were administered at the same time: one-time intraperitoneal injection of 20 mg / kg cisplatin, one group was the negative control group, and the other group was intragastrically administered paeonol (30 mg / kg) every day. mg / kg), for 3 consecutive days. On the fourth day, the mice were sacrificed after taking heart blood and kidney ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com