Organic AIE photosensitive probe with mitochondrial targeting and preparation method and application thereof
A mitochondrial and organic technology, applied in the field of anti-tumor drugs, can solve the problems of unknown mechanism, uneven induction effect, immunogenic death, etc., and achieve the effect of improving immunogenicity and good anti-tumor immune response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
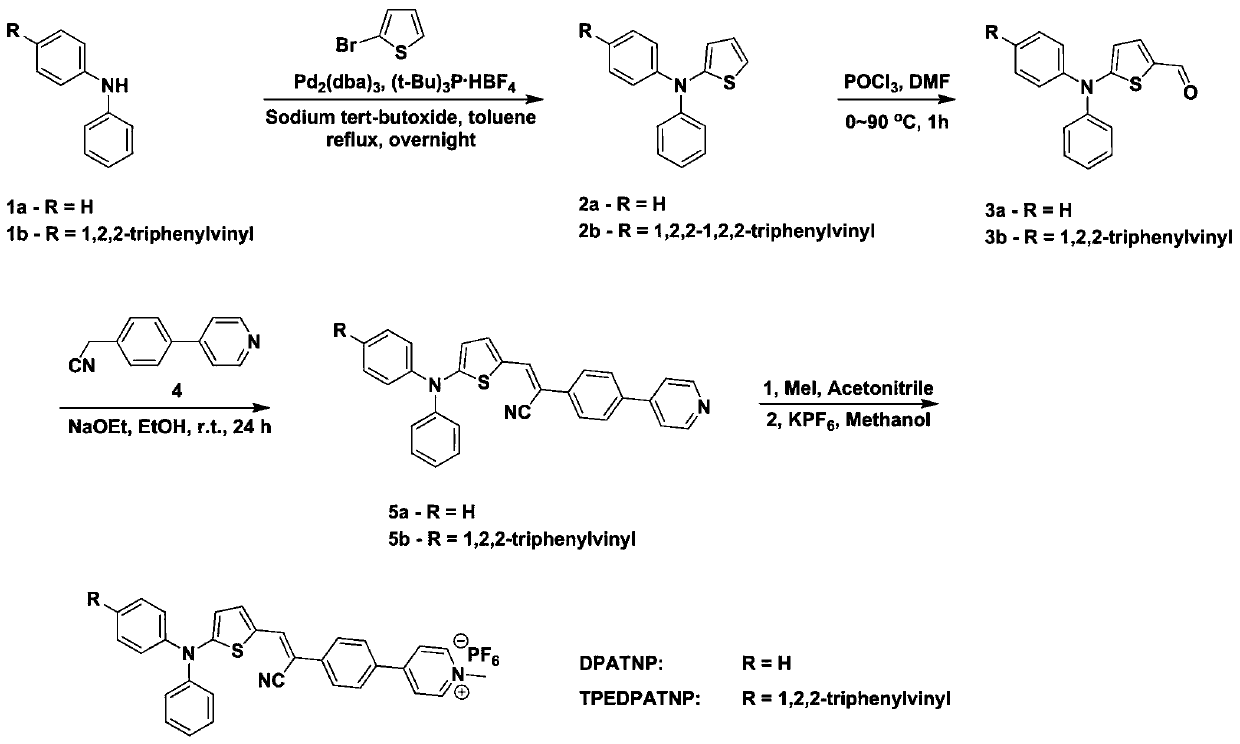
[0036] The invention provides the preparation method of the organic AIE photosensitive probe with mitochondrial targeting, comprising the following steps:
[0037] 1) Under the protection of argon, compound 1, 2-bromothiophene, tri-tert-butylphosphine tetrafluoroborate, sodium tert-butoxide, Pd 2 (dba) 3 Mixing, the obtained mixture is mixed with an organic solvent to carry out a substitution reaction to obtain a compound 2 having a structure shown in formula III;
[0038] 2) Under the condition of ice bath, mix the DMF solution containing the compound 2 and phosphorus oxychloride, naturally warm up to 23-27°C, then gradually heat to 80-100°C for substitution reaction for 1-1.5h, to obtain Compound 3 of the structure shown in formula IV;
[0039] 3) Under the condition of ice bath and under the protection of argon, the absolute ethanol solution in which the compound 3 and the compound 4 having the structure shown in formula V are dissolved is mixed with sodium ethoxide, and ...
Embodiment 1
[0064] Compound 1a (1g, 5.92mmol, 1.0eq.), 2-bromothiophene (1.45g, 8.88mmol, 1.5eq.), tri-tert-butylphosphine tetrafluoroborate (70mg, 0.24mmol, 0.04eq.) , sodium tert-butoxide (853mg, 8.88mmol, 1.5eq.) and Pd 2 (dba) 3 (108mg, 0.12mmol, 0.02eq) were mixed under the protection of argon, anhydrous toluene (toluene, tetrahydrofuran, DMF and other solvents) (30mL) was added, and the substitution reaction was carried out at 110°C for 16 hours. After cooling to room temperature, the reaction mixture was filtered through celite, and 30 mL of water and 200 mL of chloroform were added to the filtrate for liquid separation. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to remove the solvent. The crude compound 2a was directly used in the next reaction without further purification.
[0065] To a solution of compound 2a (1.5 g, crude) in DMF (25 mL) was added dropwise phosphorus oxychloride (2.3 g, 14.8 mmol, 2.5 eq.) under ic...
Embodiment 2
[0072] Compound 1b (1g, 2.36mmol, 1.0eq.), 2-bromothiophene (577mg, 3.54mmol, 1.5eq.), tri-tert-butylphosphine tetrafluoroborate (30mg, 0.097mmol, 0.04eq.), Sodium tert-butoxide (340mg, 3.54mmol, 1.5eq.) and Pd 2 (dba) 3 (45mg, 0.049mmol, 0.02eq) were mixed under the protection of argon, 20mL of anhydrous toluene was added, and the reaction was carried out at 110°C for 16h. After cooling to room temperature, the reaction mixture was filtered through celite, and 30 mL of water and 200 mL of chloroform were added to the filtrate for liquid separation. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to remove the solvent. The crude product was directly used in the next reaction without purification.
[0073] In an ice bath, phosphorus oxychloride (757 mg, 4.95 mmol, 2.5 eq.) was added dropwise to compound 2b dissolved in DMF (25 mL). After the dropwise addition, it was naturally raised to room temperature, and then gradu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com