Polysubstituted benzothienoisoquinoline, derivative and synthesis method thereof
A technology of benzothiophene and a synthesis method is applied in the field of organic compound synthesis, and achieves the effects of simple reaction system, convenient experimental operation and scientific process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-45
[0061] The synthetic method of multi-substituted benzothienoisoquinolines and derivatives comprises the following steps:
[0062] Step 1: Add ketoxime ester compound (see Table 1 for specific substance), o-chlorobenzaldehyde compound (see Table 1 for specific substance) and sulfur powder and alkali into the reaction vessel, add copper catalyst (see Table 1 for specific substance) Add organic solvent (see Table 1 for specific substances) and mix in the reaction vessel;
[0063] Step 2: The reaction vessel is evenly heated (such as oil bath heating) to the temperature described in Table 1, and the acetophenone oxime ester compound, o-chlorobenzaldehyde compound and sulfur powder are reacted in the solvent, and continue Table 1 the time stated in;
[0064] Step 3: Purify after the reaction is completed.
[0065] Table 1: Molar ratio, reaction temperature and reaction time of formaldehyde compound, ketoxime ester compound, sulfur powder, copper catalyst and alkali in embodiment 1-...
Embodiment 1
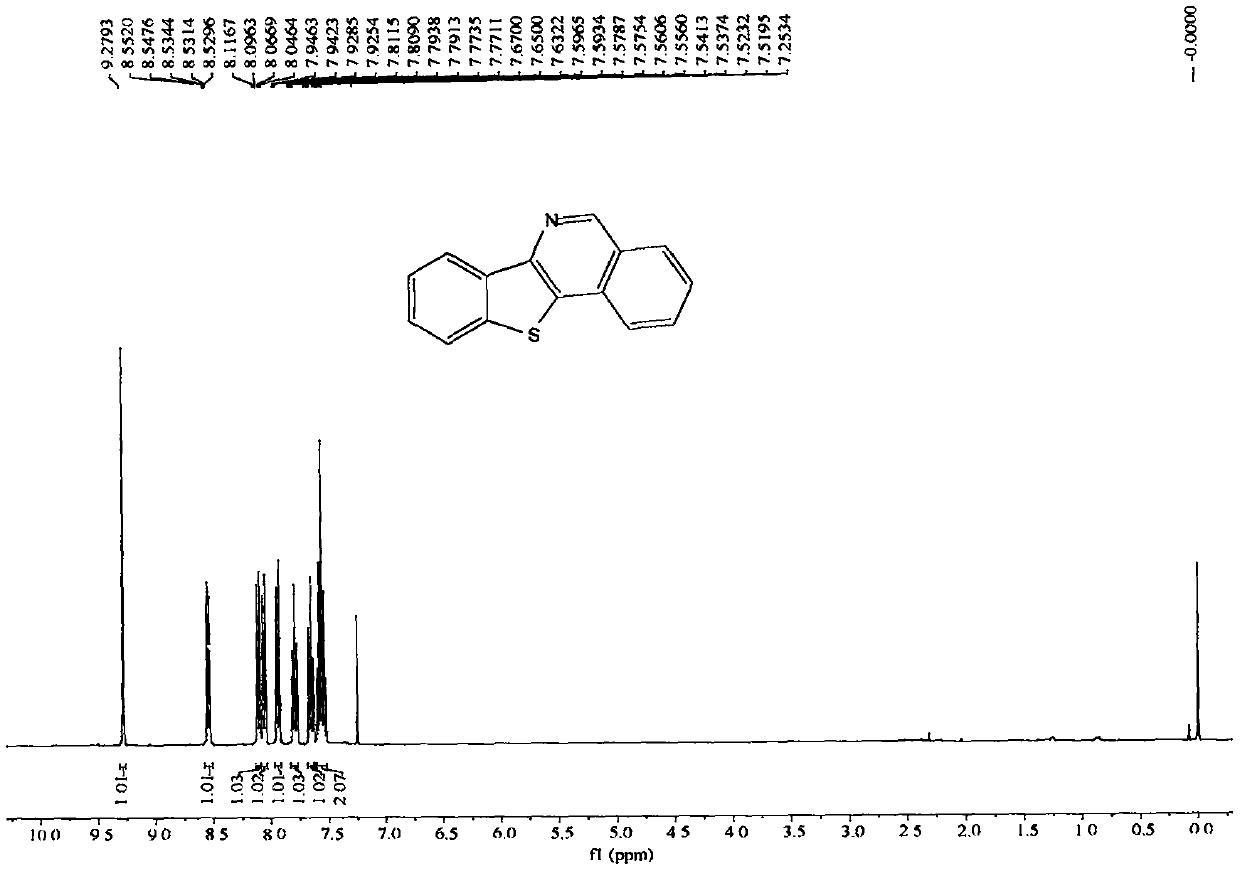
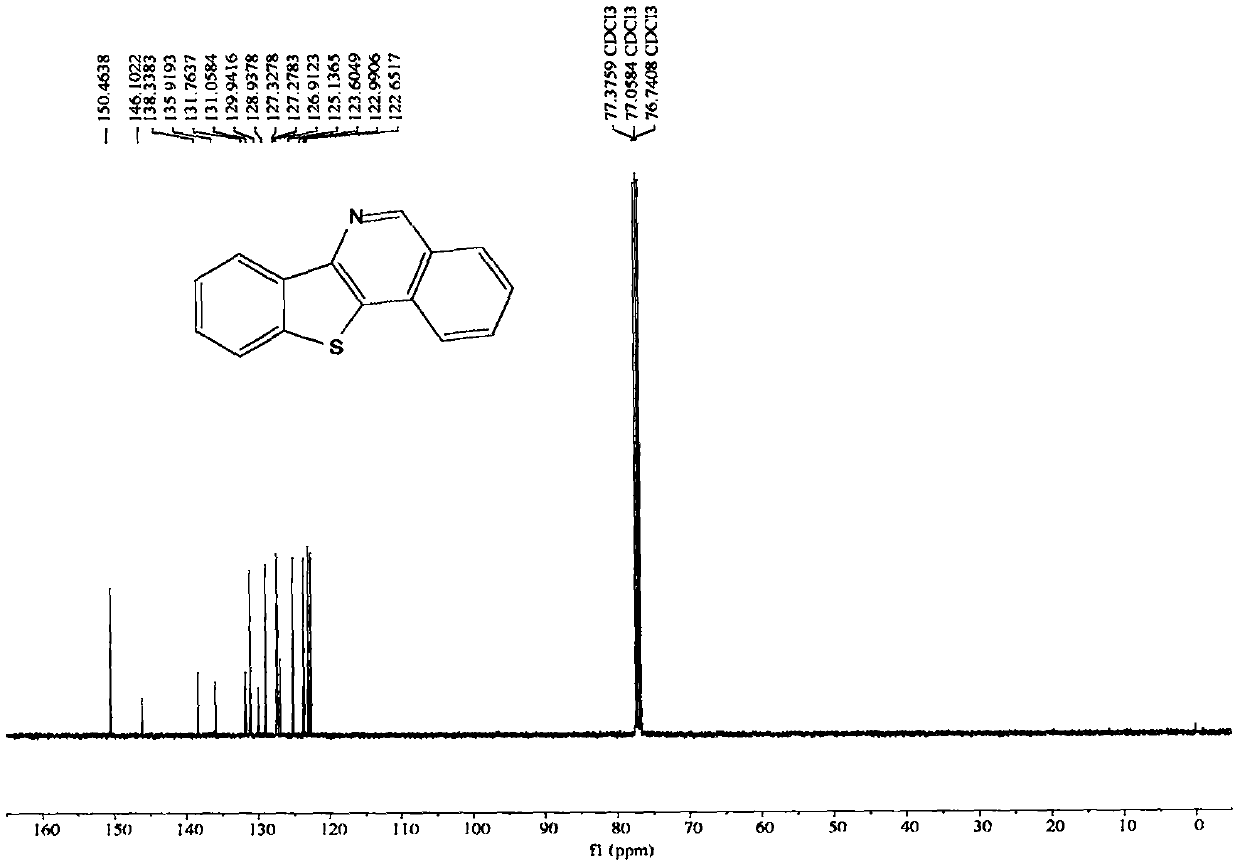
[0071] The nuclear magnetic data of embodiment 1 product is as follows:
[0072] 1H NMR (400MHz, CDCl3, ppm) δ9.28(s, 1H), 8.58-8.51(m, 1H), 8.11(d, J=8.1Hz, 1H), 8.06(d, J=8.2Hz, 1H) , 7.94(dd, J=6.9, 1.4Hz, 1H), 7.83-7.77(m, 1H), 7.66(t, J=7.6Hz, 1H), 7.56(pd, J=7.1, 1.4Hz, 2H). 13C NMR (100MHz, CDCl3, ppm) δ150.5, 146.1, 138.3, 135.9, 131.8, 131.1, 129.9, 128.9, 127.3, 127.3, 126.9, 125.1, 123.6, 123.0, 122.7.
[0073] The nuclear magnetic data of embodiment 2 product is as follows:
[0074] 1H NMR (400MHz, CDCl3, ppm) δ9.27(s, 1H), 8.43(d, J=8.1Hz, 1H), 8.11(d, J=8.2Hz, 1H), 8.05(d, J=8.2Hz , 1H), 7.83-7.77(m, 1H), 7.73(s, 1H), 7.65(t, J=7.3Hz, 1H), 7.39(d, J=8.1Hz, 1H), 2.55(s, 3H) .13C NMR (100MHz, CDCl3, ppm) δ150.1, 145.9, 138.6, 137.7, 133.4, 131.9, 131.1, 129.4, 128.9, 127.0, 126.8, 126.6, 123.5, 122.9, 122.3, 21.8.
Embodiment 3
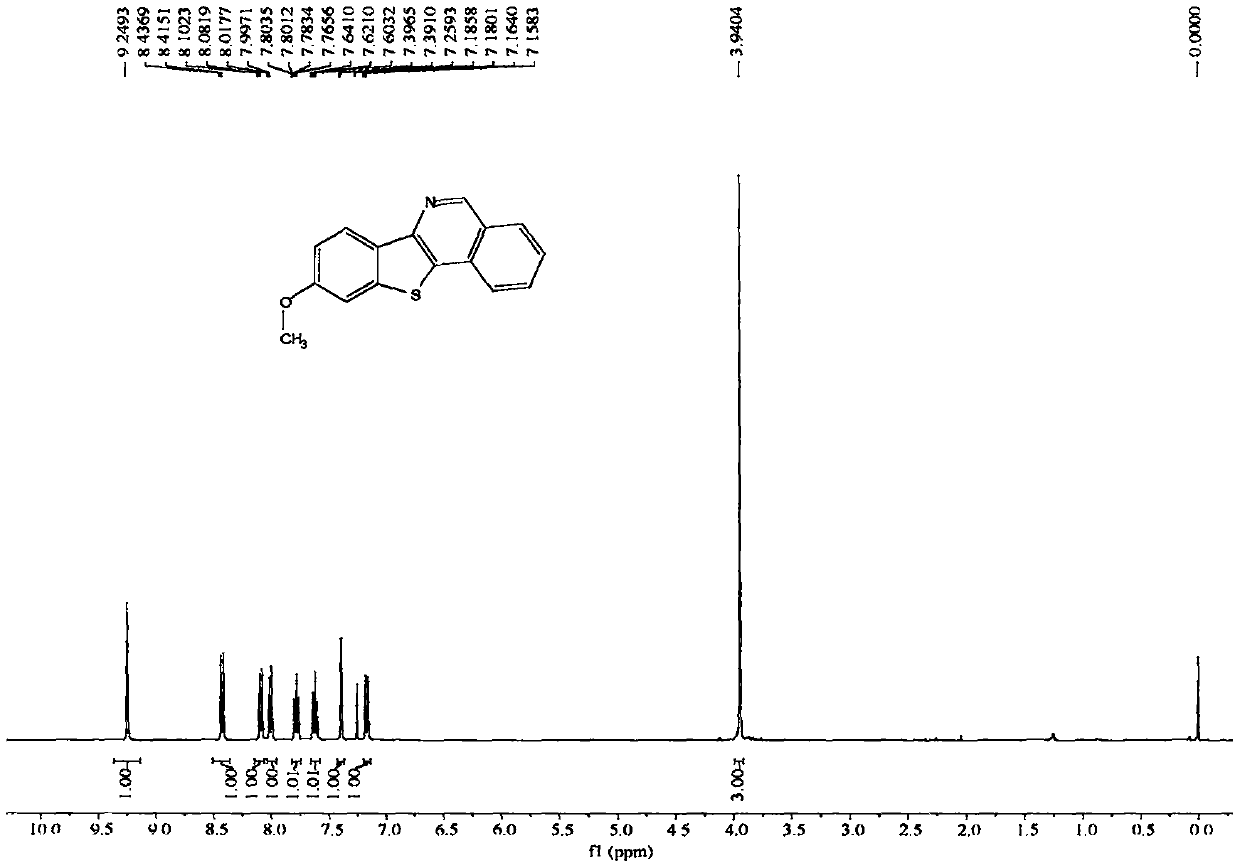
[0075] The NMR data of embodiment 3 product are as follows:
[0076] 1H NMR (400MHz, CDCl3, ppm) δ9.25(s, 1H), 8.43(d, J=8.7Hz, 1H), 8.09(d, J=8.2Hz, 1H), 8.01(d, J=8.2Hz , 1H), 7.79(dd, J=11.2, 4.0Hz, 1H), 7.62(t, J=7.6Hz, 1H), 7.39(d, J=2.2Hz, 1H), 7.17(dd, J=8.7, 2.3Hz, 1H), 3.94(s, 3H).13C NMR (100MHz, CDCl3, ppm) δ159.7, 150.1, 145.7, 140.0, 131.9, 131.1, 129.3, 129.0, 128.5, 126.8, 126.3, 123.4, 123.3, 114.4, 106.0, 55.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com