Natural sulfide mineral-based material as well as preparation method and application thereof
A technology of base materials and minerals, applied in the field of preparation, natural sulfide mineral base materials, can solve the problems of hindering large-scale production and wide application, small specific surface area of natural sulfide minerals, poor mercury adsorption capacity, etc., to achieve abundant reserves and low cost. , the effect of improving mercury removal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Se 2- Preparation of precursor solution: Mix 20 mL of deionized water and 20 mL of hydrazine hydrate (80wt%), adjust the mixed solution to be alkaline with NaOH, and dissolve 0.016 mol, 0.032 mol, 0.064 mol, and 0.128 mol of selenium powder in the above mixture. Solution
[0035] (2) The natural pyrrhotite (Fe 1-x S) with Se 2- The molar ratio of 1:4, 1:8, 1:16, 1:32 is added with Se 2- Precursor, stir for 30 min;
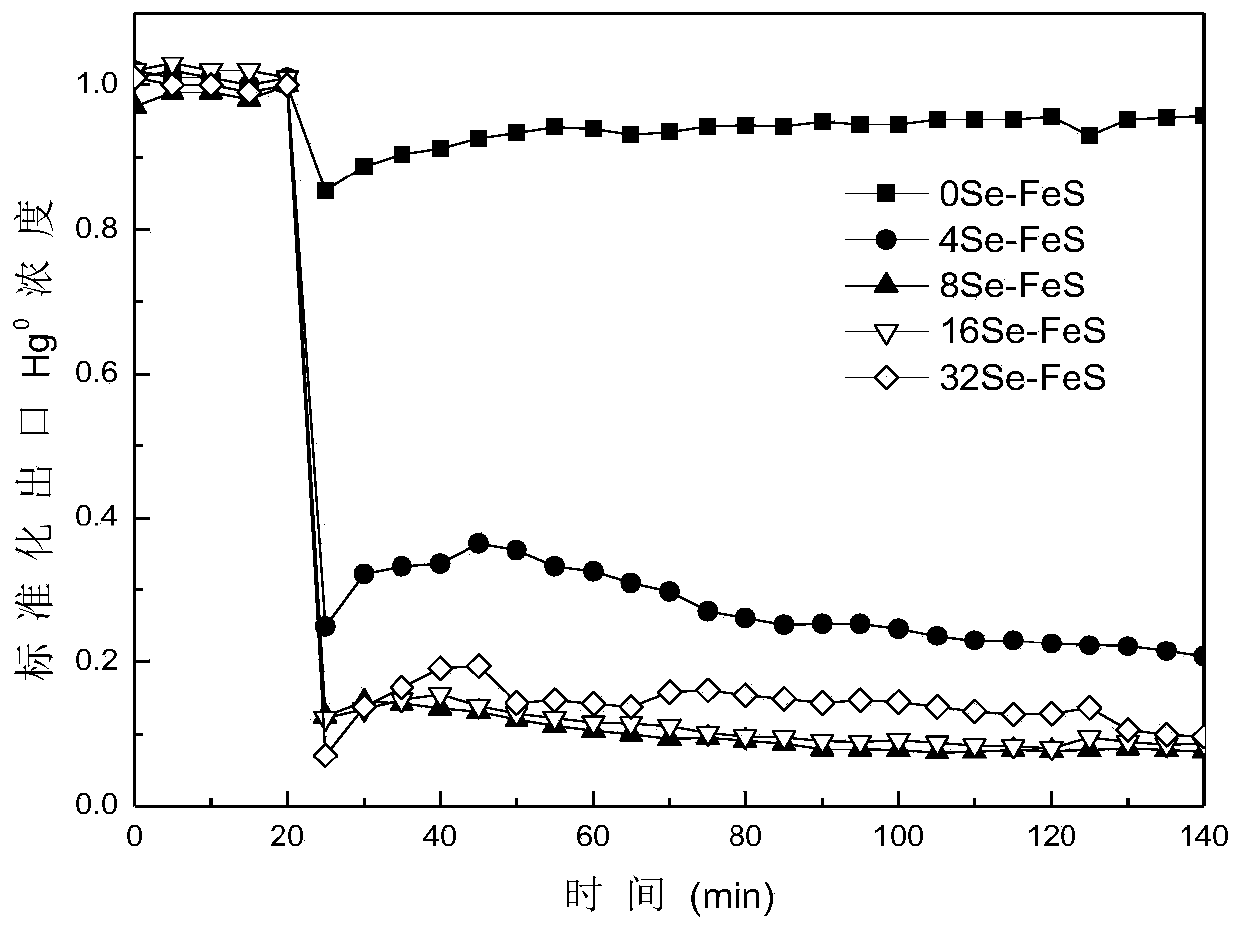
[0036] (3) Combine the adsorbent in step (2) with Se-containing 2- The precursor liquid was separated, and the separated adsorbent was washed 5 times with deionized water, dried in an oven at 60 ℃ for 12 hours, and passed through a 100-mesh sieve to obtain a new modified metal sulfide mercury adsorbent, which was recorded as 4Se-FeS and 8Se- FeS, 16Se-FeS, 32Se-FeS, no Se 2- Natural pyrrhotite (Fe 1-x S) Denoted as 0Se-FeS;
[0037] (4) Weigh 100 mg of adsorbent and place it in a cylindrical quartz glass fixed bed reactor with a length of 10 cm and an inner di...
Embodiment 2
[0039] (1) Se 2- Preparation of precursor solution: Mix 20 mL of deionized water and 20 mL of hydrazine hydrate (80wt%), adjust the mixed solution to be alkaline with NaOH, and dissolve 0.032 mol of selenium powder in the above mixed solution;
[0040] (2) The natural pyrrhotite (Fe 1-x S) with Se 2- The molar ratio of 1:8 is added with Se 2- Precursor, stir for 30 min;
[0041] (3) Combine the adsorbent in step (2) with Se-containing 2- The precursor liquid was separated, and the separated adsorbent was washed with deionized water 5 times, dried in an oven at 60 ℃ for 12 h, and passed through a 100-mesh sieve to obtain a new modified metal sulfide mercury adsorbent, denoted as 8Se-FeS;
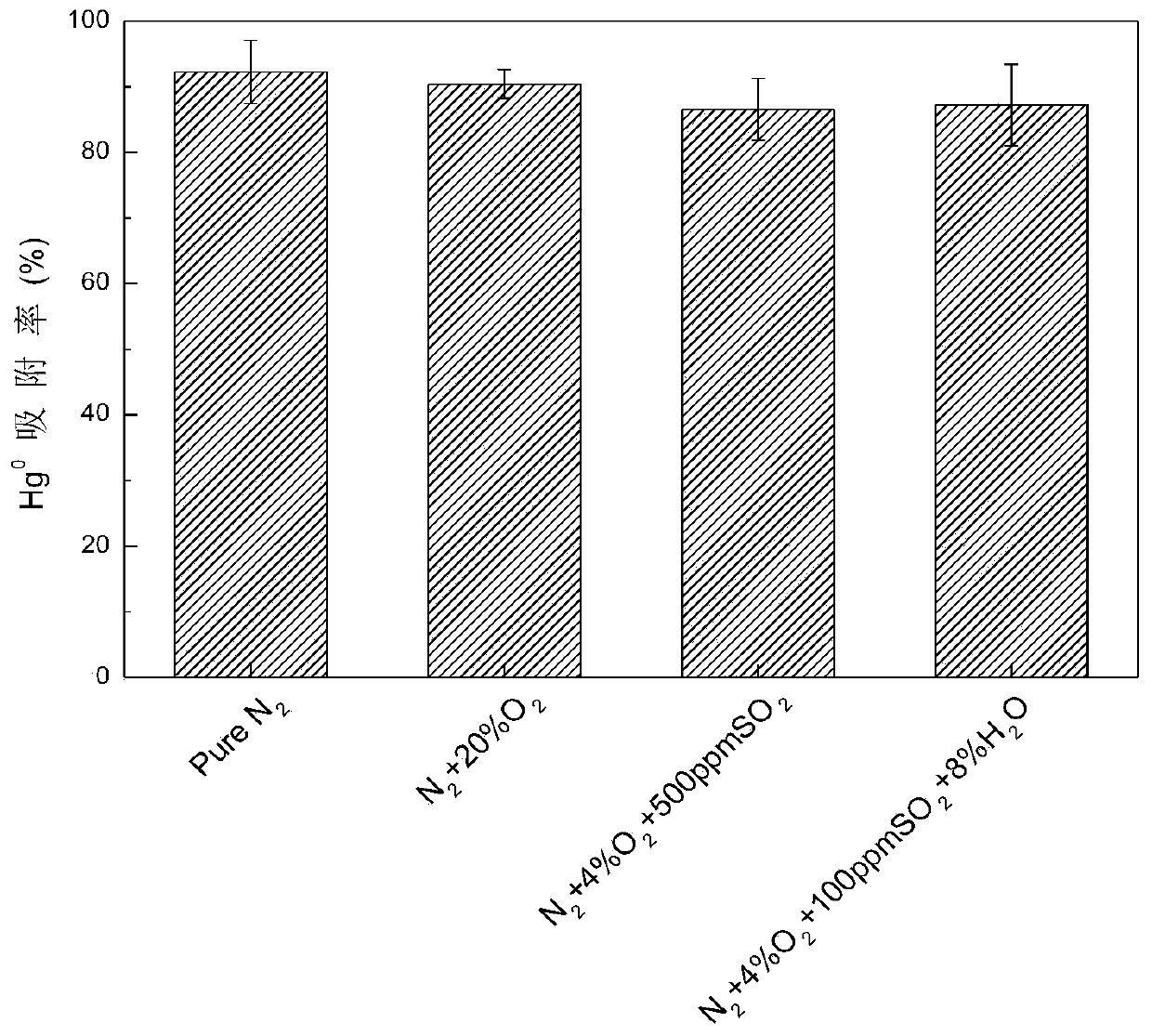
[0042] (4) Weigh 100 mg of 8Se-FeS and place it in a cylindrical quartz glass fixed bed reactor with a length of 10 cm and an inner diameter of 1 cm. Produce elemental mercury, Hg through the mercury permeation tube 0 Initial concentration 80 μg / m 3 , Respectively add simulated flue gas as N 2 , N 2...
Embodiment 3
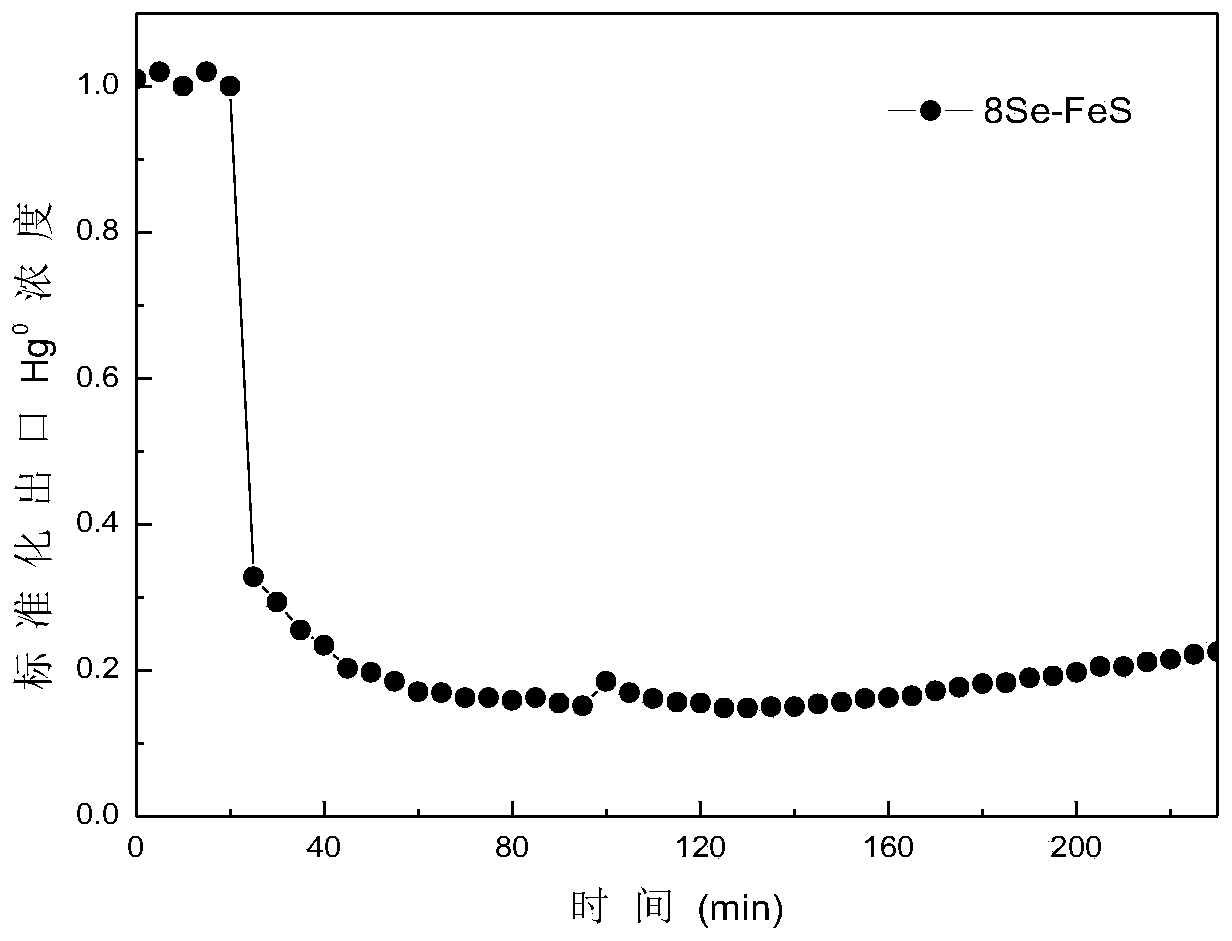
[0044] (1) The 8Se-FeS modified in Example 1 is added in a fixed bed after the electrostatic precipitator (including wet electrostatic precipitator), Hg 0 The emission is 3.77 mg / m 3 , The flue gas volume is 18000 m 3 / h, the fixed bed 8Se-FeS load is 2 kg, divided into 4 layers of load, Hg 0 The removal rate reaches 90%, further adsorption deactivates the adsorbent; the deactivated adsorbent removes Hg 0 The effect picture is like image 3 Shown.
[0045] (2) Place the deactivated 8Se-FeS in a heating furnace, heat it under the protection of nitrogen at 400 ℃ for 1 h, and then cool the adsorbent to room temperature by natural cooling to obtain the regenerated adsorbent. The high-concentration mercury-containing gas released during the adsorbent regeneration process adopts the condensation method for mercury resource recovery, and the tail gas is discharged after mercury removal by the adsorbent;
[0046] (3) After heat treatment, the 8Se-FeS is combined with Se again 2- The molar r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com