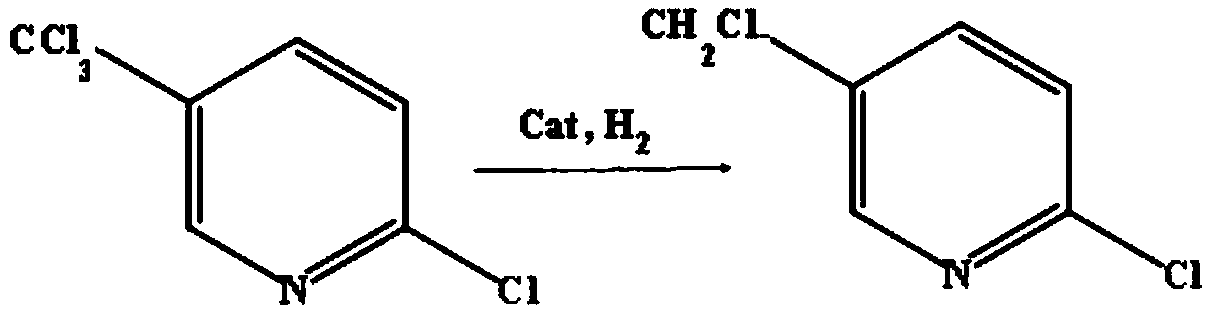
Method for preparing 2-chloro-5-chloromethylpyridine
A technology of chloromethylpyridine and trichloromethylpyridine, which is applied in the field of preparation of 2-chloro-5-chloromethylpyridine, can solve the problems of poor selectivity of chlorination reaction and low yield of target product, and realize recycling , The synthesis method is green and environmentally friendly, and the process control is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The method for preparing 2-chloro-5-chloromethylpyridine in this example uses 2-chloro-5-trichloromethylpyridine as a raw material for selective hydrogenation dechlorination reaction to obtain 2-chloro-5-chloromethylpyridine pyridine;
[0036] The catalyst of the selective hydrodechlorination reaction is Pd / C catalysis, and the Pd / C catalyst includes carrier activated carbon and noble metal palladium loaded on the carrier activated carbon, and the mass percentage of noble metal Pd in the Pd / C catalyst is 3%;
[0037] The acid-binding agent of the selective hydrodechlorination reaction is sodium phosphate;
[0038] The organic solvent of the selective hydrodechlorination reaction is methyl acetate;
[0039] The preparation method comprises the following steps:
[0040] Step 1, dissolving sodium phosphate in water to obtain an aqueous sodium phosphate solution with a mass content of 30%, 1300g methyl acetate is placed in an autoclave, and 1000g2-chloro-5-trichloromet...
Embodiment 2
[0051] This embodiment is the same as embodiment 1, wherein the difference is that the acid-binding agent of the selective hydrodechlorination reaction is sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, triethylamine, triethanolamine, 2 -Hydroxyethylamine or ammonia gas.
[0052] In this example, the yield of 2-chloro-5-chloromethylpyridine is 87.1%-87.3%, and the gas chromatography purity of 2-chloro-5-chloromethylpyridine is 98.4%-98.5%. In this embodiment, the recycled catalyst was recycled 5 times, and the yield and purity of 2-chloro-5-chloromethylpyridine remained basically unchanged during the recycled process.
Embodiment 3
[0054] The method for preparing 2-chloro-5-chloromethylpyridine in this example uses 2-chloro-5-trichloromethylpyridine as a raw material for selective hydrogenation dechlorination reaction to obtain 2-chloro-5-chloromethylpyridine pyridine;
[0055] The catalyst of the selective hydrodechlorination reaction is Pd / C catalysis, and the Pd / C catalyst includes carrier activated carbon and noble metal palladium loaded on the carrier activated carbon, and the mass percentage of noble metal Pd in the Pd / C catalyst is 10%;
[0056] The acid-binding agent of the selective hydrodechlorination reaction is triethanolamine;
[0057] The organic solvent of the selective hydrodechlorination reaction is ethyl acetate;
[0058] The preparation method comprises the following steps:
[0059] Step 1, 2500g ethyl acetate is placed in autoclave, drop into 1000g2-chloro-5-trichloromethylpyridine, 1gPd / C catalyst, 552g triethanolamine and 400g pure water in described autoclave, first use Nitro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com