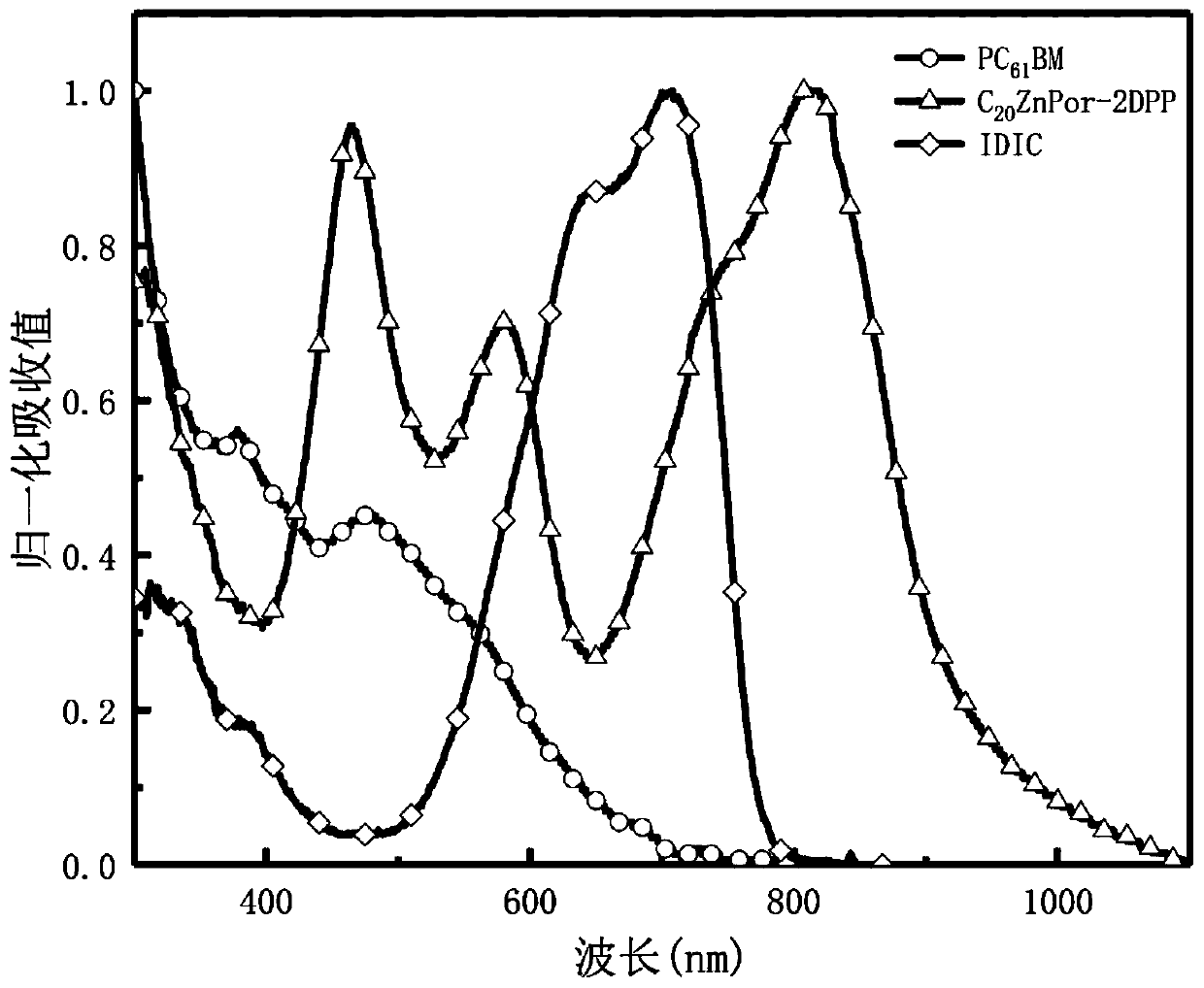
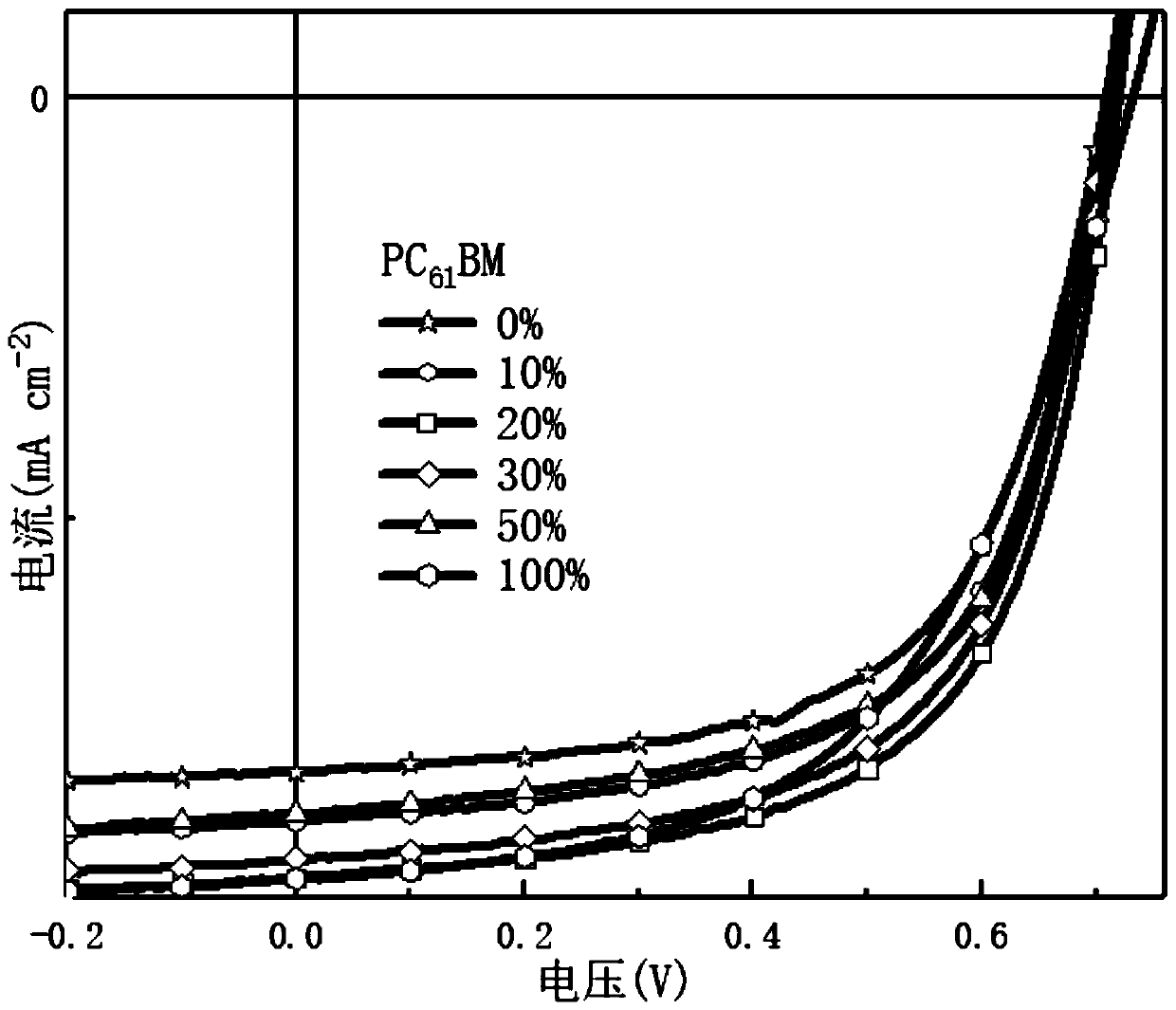
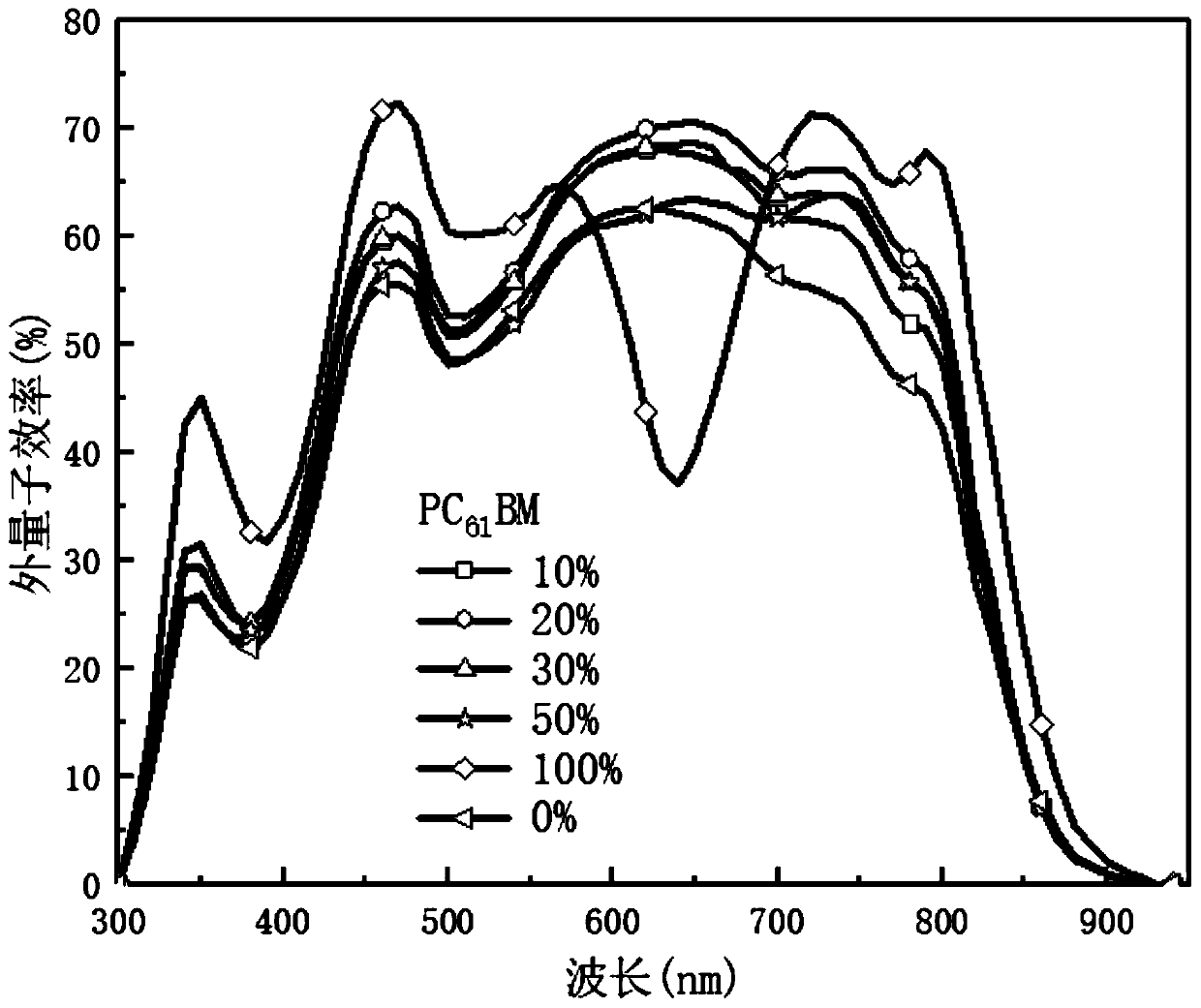
Efficient ternary organic solar cell based on porphyrin material and preparation method thereof
A technology of organic solar cells and porphyrins, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of limited number of excitons, strong molecular aggregation, low efficiency, etc., achieve high photoelectric conversion efficiency, improve morphology, Improve absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The synthetic equation of 5,15-bisbromo-10,20-bis(octyldodecyl)zinc porphyrin is:
[0066]
[0067] Dissolve 5,15-bis(3,4-bis(octyldodecyl)porphyrin (500mg, 0.59mmol) in 100mL of chloroform, add 1mL of pyridine, protect from light and fully dissolve, then add bromo Succinimide (NBS) (221mg, 1.24mmol), reacted at 0°C for 30 minutes, then continued to react overnight at room temperature, and finally quenched the reaction with acetone. After the reaction was completed, add water, extract with chloroform, anhydrous sulfuric acid Sodium is dried, spin-dried the solvent and dissolved in 50mL of chloroform solution, then added 10mL of zinc acetate methanol solution (540mg, 2.95mmol of zinc acetate dissolved in 10mL of methanol solvent), and refluxed for 2 hours in the dark. After the reaction was completed, wash with water , and dried with anhydrous sodium sulfate, spin-dried the solvent, and purified by silica gel column chromatography to obtain a purple solid. 1 H NMR (4...
Embodiment 2
[0070] The synthesis of 5,15-bis(trimethylsilylacetylene)-10,20-bis(octyldodecyl)zinc porphyrin is as follows:
[0071]
[0072] In a 100mL two-necked round-bottomed flask, add 5,15-bisbromo-10,20-bis(octyldodecyl)zinc porphyrin (400mg, 0.37mmol), 40mL tetrahydrofuran and 20mL triethylamine, and nitrogen After 30 minutes, bis(triphenylphosphine)palladium dichloride (26mg, 0.037mmol), copper iodide (CuI) (7.18mg, 0.037mmol) and trimethylsilylacetylene (368mg, 3.7mmol) were added, Protected from light, the reaction was stirred at room temperature for three days. After the reaction was completed, it was extracted with chloroform, washed with water, dried over anhydrous sodium sulfate, and then subjected to column chromatography on silica gel / (dichloromethane / methanol=20 / 1 as eluent), and spin-dried to obtain a purple-green solid. 1 HNMR (400MHz, Chloroform-d) δ9.74 (dq, J = 9.8, 5.0Hz, 4H), 9.63 (m, 2H), 5.20-5.12 (m, 2H), 2.98-2.70 (dq, J = 18.5, 5.5Hz, 4H), 2.60(dq, J=18.5...
Embodiment 3
[0074] Synthesis of final product ZnPor-2DPP
[0075]
[0076] Dissolve 5,15-bis(trimethylsilylacetylene)-10,20-bis(octyldodecyl)zinc porphyrin (100 mg, 0.091 mmol) in 10 mL of tetrahydrofuran solution, add tetrabutyl fluoride Ammonium (0.23 mL, 1M in THF), the reaction was stirred at room temperature for 5 minutes, and water was added to quench the reaction. It was extracted with chloroform, dried over anhydrous sodium sulfate, and spin-dried. The impurities were separated through a gel column, and a green solid was obtained by spin-drying.
[0077] Under the protection of argon, 5,15-bis(acetylene)-10,20-bis(octyldodecyl)zinc porphyrin (80mg, 0.084mmol) was added to a 50mL two-necked round-bottomed flask, 3- (5-Bromo-2-thienyl)-2,5-bis(2-ethylhexyl)-2,5-dihydro-6-(2-thienyl)pyrrolo[3,4-C]pyrrole- 1,4-Diketone (152mg, 0.25mmol), anhydrous toluene (20mL), triethylamine (10mL), cuprous iodide (1.6mg, 0.0084mmol) and tetrakis(triphenylphosphine) palladium (9.7mg ,0.0084mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com