N-heterocyclic carbene palladium complex crystal, synthetic method thereof, and application thereof in preparation of amide compounds
A technology for amide compounds and aniline compounds, which is applied in the field of azacarbene palladium complex crystals to achieve the effects of high electron affinity, large bond energy and increasing electron density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
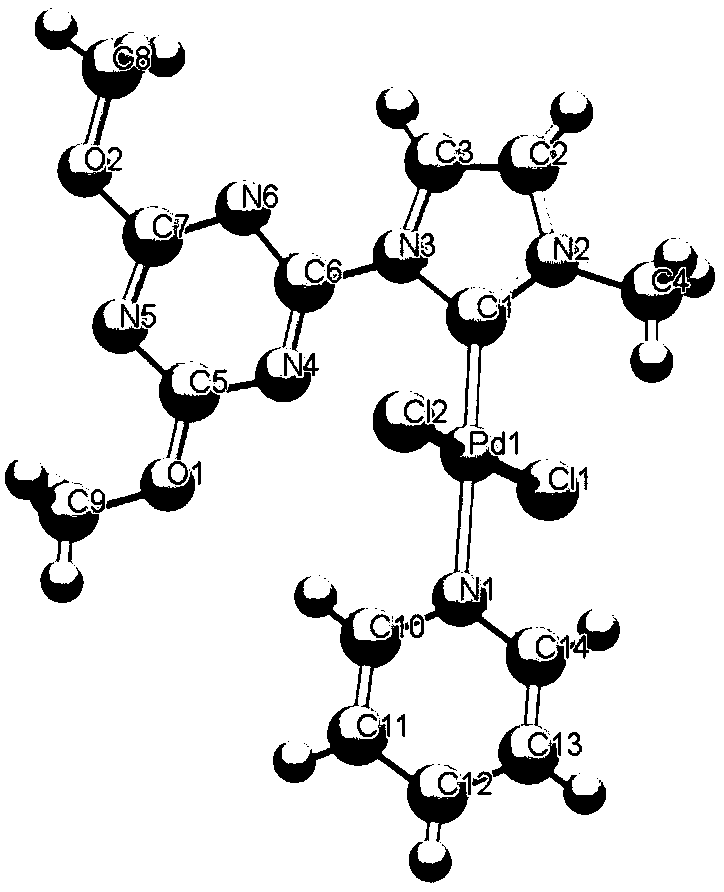
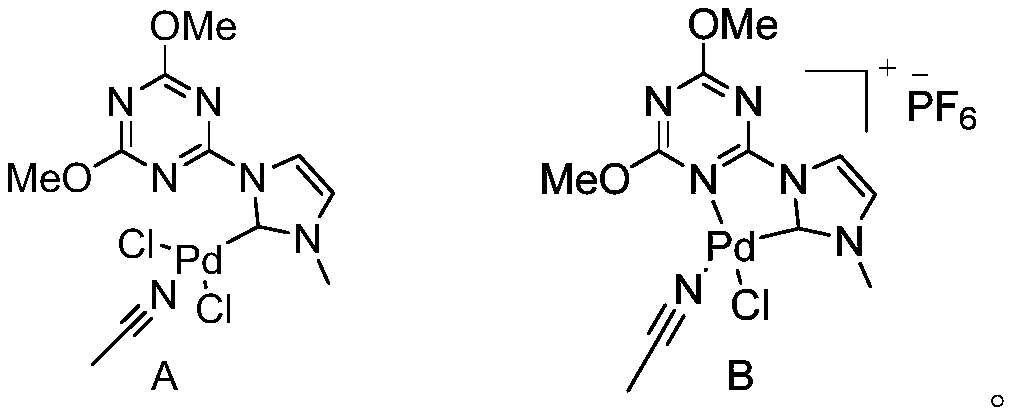
[0022] Synthesis of the azacarbene palladium complex crystal A with the following structural formula
[0023]
[0024] With 367.19mg (1mmol) azacarbene ligand shown in formula I and 115.9mg (0.5mmol) Ag 2 O was dissolved in 5 mL of acetonitrile, and 259.43 mg (1 mmol) of Pd(CH 3 EN) 2 Cl 2 , stirred and reacted at room temperature for 1 hour, after the reaction was completed, assisted filtration with diatomaceous earth, and recrystallized with a mixture of acetonitrile and n-hexane with a volume ratio of 1:3 to obtain azacarbene palladium complex crystal A , the yield is 55%, and the X-ray single crystal structure is shown in figure 1 As shown, it belongs to the triclinic crystal system, the space group P-1, and the unit cell parameters are: α=75.1030(10), β=86.4900(10), γ=88.6250(10), Z=2, Pd01-Cl02=2.3156(6), Pd01-Cl03=2.3285(6), Pd01-N006=2.079(2), Pd01-C00B=1.934(3), N008-C00E=1.333(3), N008 -C00F=1.328(3), N009-C00E=1.333(3), N009-C00G=1.334(3), Cl02-Pd01-Cl0...
Embodiment 2
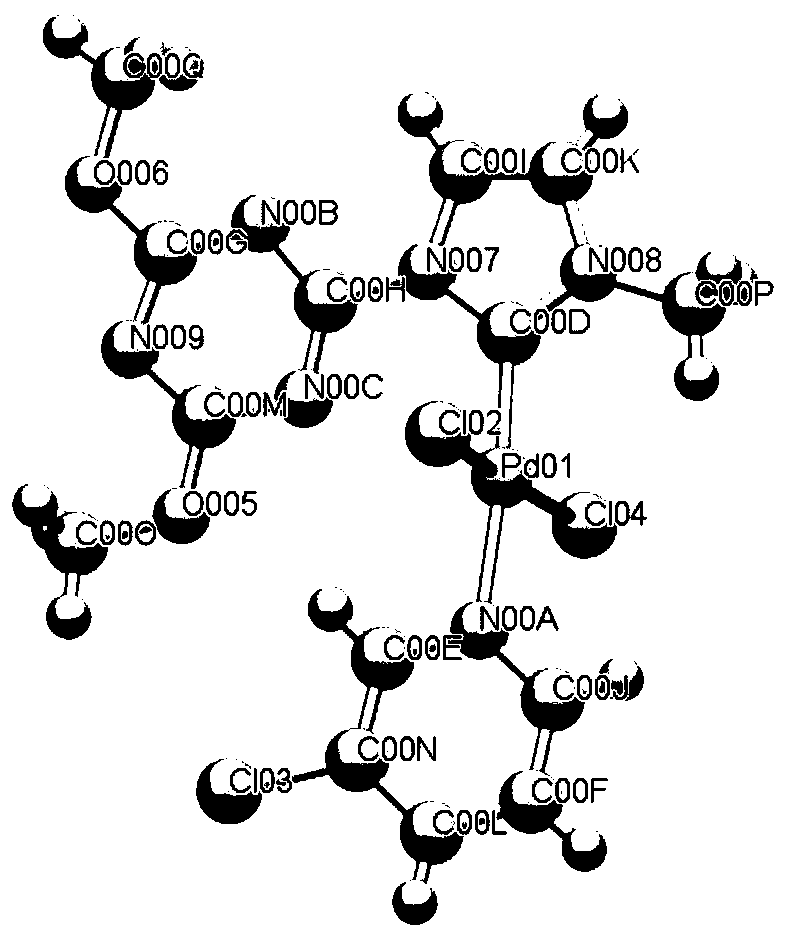
[0026] Synthesis of the azacarbene palladium complex crystal B with the following structural formula
[0027]
[0028] In this embodiment, the azacarbene ligand shown in formula I in Example 1 is replaced with the azacarbene ligand shown in formula II in equimolar form, and the other steps are the same as in Example 1 to obtain the azacarbene palladium complex B, which produces The ratio is 57%, and the X-ray single crystal structure diagram is as follows figure 2 As shown, it belongs to the monoclinic crystal system, the P21 / n space group, and the unit cell parameters are: α=90°, β=103.435(4)°, γ=90°, Z=4, N005-Pd01-Cl02=175.49(8), N00B-Pd01-Cl02=85.99(10), N00B-Pd01-N005=98.45(12), C00J-Pd01-Cl02=96.53(10), C00J- Pd01-N005 = 79.00 (13), C00J-Pd01-N00B = 177.01 (14). The NMR data of the complex crystal are: 1 H NMR (600MHz, CD 3 CN)δ7.85(d,J=2.3Hz,1H),7.23(d,J=2.3Hz,1H),4.20(s,6H),4.12(s,3H),1.97(s,3H),1.94 (dt,J=4.9,2.5Hz,5H). 13 C NMR (101MHz, DMSO) δ177.61(s),...
Embodiment 3
[0030]The following structural formula is prepared: N-phenylbenzamide
[0031]
[0032] To a 20 mL reaction tube, add 0.485 mg (0.001 mmol) azacarbene palladium complex crystal A, 112 μL (1 mmol) iodobenzene, 182 μL (1.5 mmol) aniline, 278 μL (2 mmol) triethylamine, 3 mL 1,4-dioxo Hexacyclic ring, and CO gas was introduced, and the reaction was stirred for 6 hours at a CO pressure of 3 atm and a temperature of 100 ° C. After the reaction was stopped, 15 mL of dichloromethane was added, and the dichloromethane was removed by rotary evaporation, and separated by a silica gel column (the eluent was The volume ratio of dichloromethane and sherwood oil is the mixed solution of 2:1), obtains N-phenylbenzamide, and its productive rate is 100%, and the spectral data of product is: 1 H NMR (600MHz, CDCl 3 )δ7.85(s,1H),7.81-7.76(m,2H),7.57(d,J=7.8Hz,2H),7.48-7.44(m,1H),7.39(t,J=7.6Hz,2H ),7.29(dd,J=10.8,5.0Hz,2H),7.07(t,J=7.4Hz,1H); 13 C NMR (151MHz, CDCl 3 )δ164.78(s), 136.92(s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com