Preparation method of Co3O4-CuCoO2 nano-net material by using aluminum powder as sacrificial agent
A sacrificial agent, nano-mesh technology, applied in catalyst activation/preparation, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problems of complex synthesis process, easy blockage of pores, unfavorable reactions, etc. Temperature, strong applicability, and the effect of broadening the temperature window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
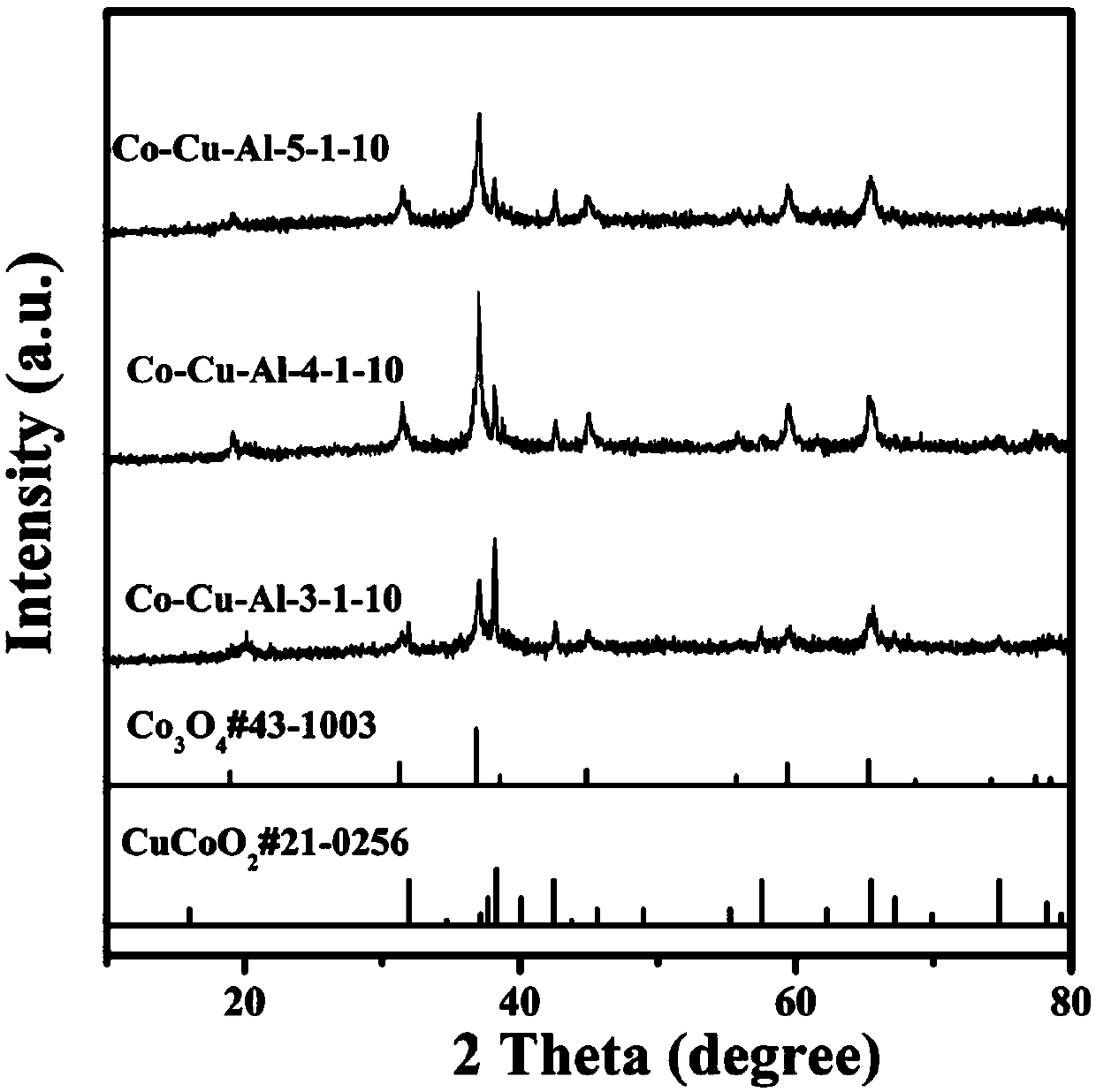
[0038] Embodiment 1: hexagonal nano network Co-Cu-Al-3-1-10 (n (Co) :n (Cu) :n (Al) =3:1:10) Preparation of materials
[0039] Dissolve 0.8731g of cobalt nitrate hexahydrate and 0.2416g of copper nitrate trihydrate in 30ml of deionized water, stir at room temperature for 30min to form a uniform solution, dissolve 5.611g of potassium hydroxide in 40ml of deionized water, and Under stirring for 30min, a uniform and transparent solution was formed, and the obtained strong alkali solution was dripped into the mixed solution of cobalt nitrate and copper nitrate obtained at a rate of 1ml / min, and 0.2698g of aluminum powder was added, which was transferred to the reaction kettle. Under the condition of ℃ for hydrothermal reaction for 20h, after cooling down to room temperature, wash with water, dry in a vacuum oven at 60℃, and then bake in a muffle furnace at 200℃ for 2h.
Embodiment 2
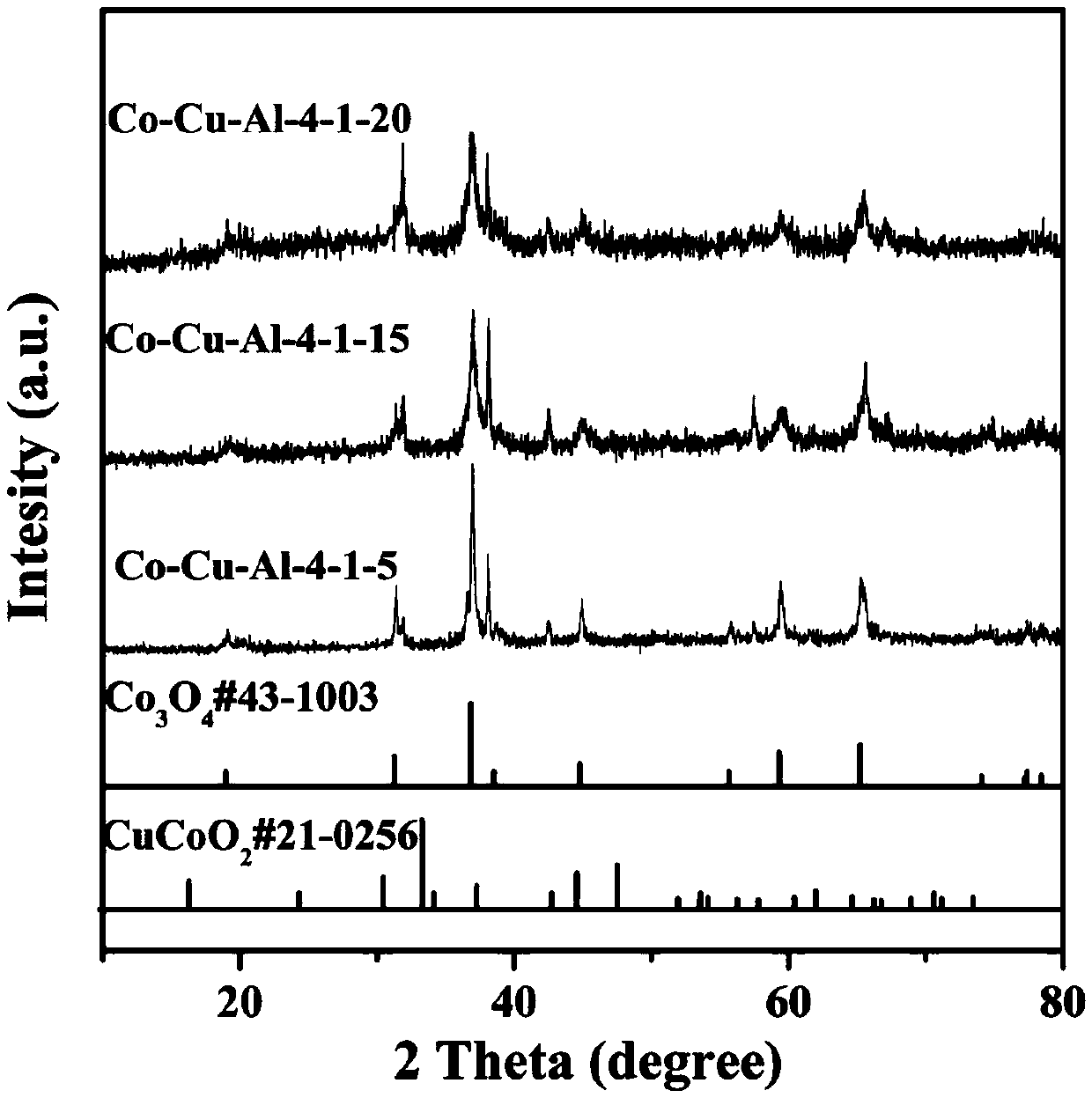
[0040] Embodiment 2: hexagonal nano network Co-Cu-Al-4-1-10 (n (Co) :n (Cu) :n (Al) =4:1:10) Preparation of materials
[0041] Dissolve 1.1642g of cobalt nitrate hexahydrate and 0.2416g of copper nitrate trihydrate in 30ml of deionized water, stir at room temperature for 30min to form a uniform solution, dissolve 5.611g of potassium hydroxide in 40ml of deionized water, and Under stirring for 30min, a uniform and transparent solution was formed, and the obtained strong alkali solution was dripped into the mixed solution of cobalt nitrate and copper nitrate obtained at a rate of 1ml / min, and 0.2698g of aluminum powder was added, which was transferred to the reaction kettle. Under the condition of ℃ for hydrothermal reaction for 20h, after cooling down to room temperature, wash with water, dry in a vacuum oven at 60℃, and then bake in a muffle furnace at 200℃ for 2h.
Embodiment 3
[0042] Embodiment 3: hexagonal nano network Co-Cu-Al-5-1-10 (n (Co) :n (Cu) :n (Al) =5:1:10) Preparation of materials
[0043] Dissolve 1.4552g of cobalt nitrate hexahydrate and 0.2416g of copper nitrate trihydrate in 30ml of deionized water, stir at room temperature for 30min to form a uniform solution, dissolve 5.611g of potassium hydroxide in 40ml of deionized water, and Under stirring for 30min, a uniform and transparent solution was formed, and the obtained strong alkali solution was dripped into the mixed solution of cobalt nitrate and copper nitrate obtained at a rate of 1ml / min, and 0.2698g of aluminum powder was added, which was transferred to the reaction kettle. Under the condition of ℃ for hydrothermal reaction for 20h, after cooling down to room temperature, wash with water, dry in a vacuum oven at 60℃, and then bake in a muffle furnace at 200℃ for 2h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com