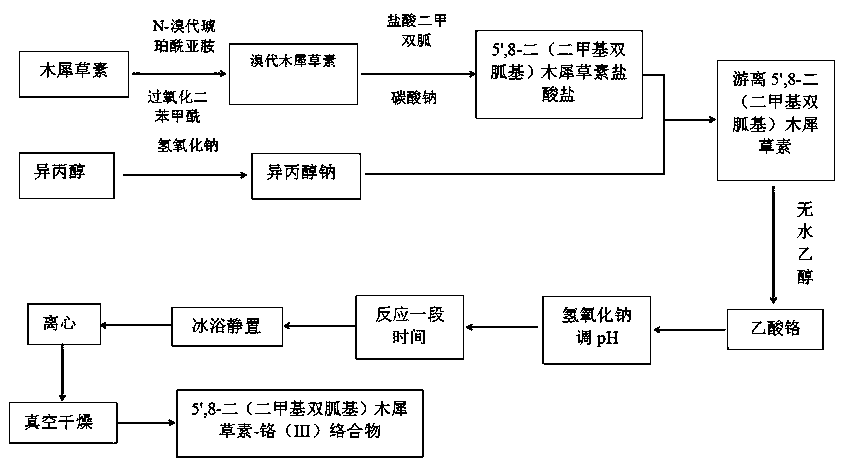
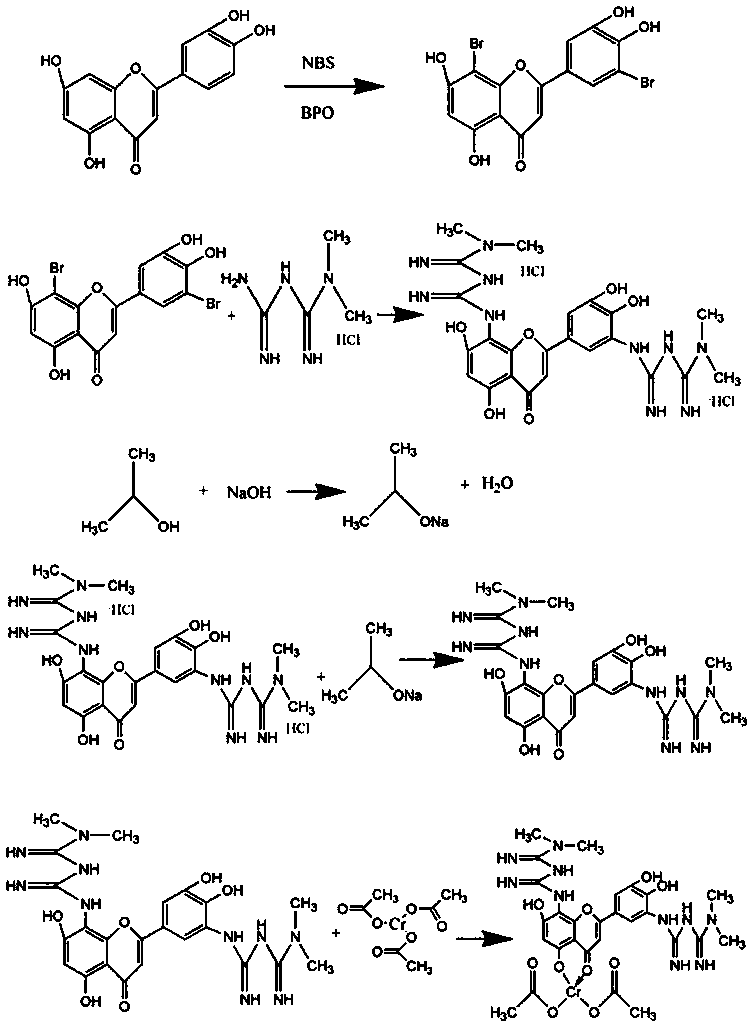
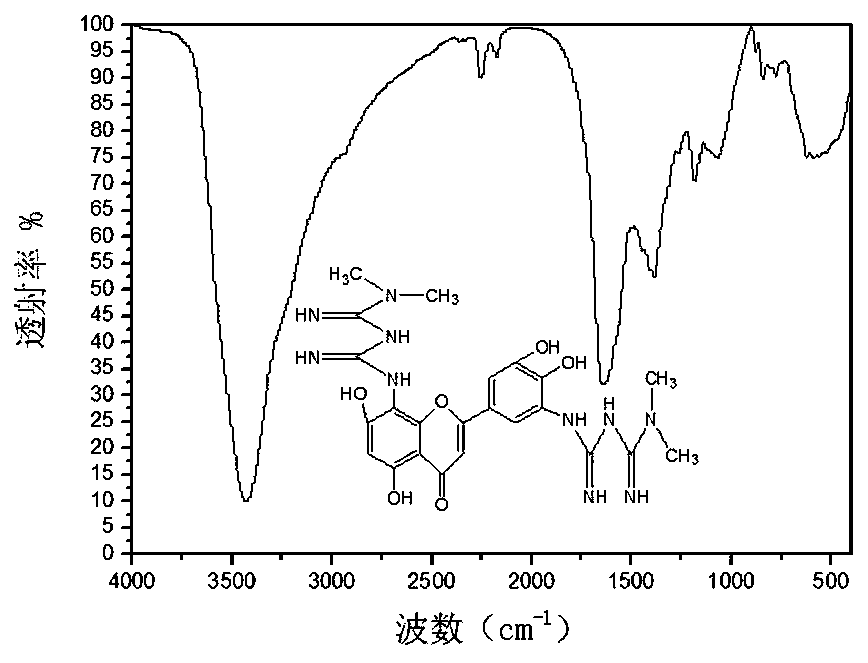
5',8-di(dimethylbiguanide)luteolin-chromium(III) complex
A technology of dimethyl biguanide and luteolin, applied in the field of biomedicine, can solve problems such as toxicity reduction and heavy metal poisoning, and achieve the effects of low cost and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 5',8-bis(dimethylbiguanidino)luteolin-chromium(III) complex:
[0033] Step a: Dissolve NBS in acetone and stir at room temperature, avoid light, dissolve as much as possible, pour into the dropping funnel for dropwise addition, dissolve luteolin in acetone, add BPO, put the dropping funnel under ice bath Start to add slowly, heat to 90°C after the dropwise addition, and react for 18 hours. After the reaction, carry out vacuum distillation to remove acetone, wash with deionized water several times, and freeze-dry to obtain bromoluteolin, wherein n(luteolin):n(NBS)=1:1.5, m(BPO):m(luteolin)=5%;
[0034] Step b: adding the bromoluteolin prepared in step a into a three-necked flask, using absolute ethanol as a solvent, sodium carbonate as a base, potassium iodide as a catalyst, adding metformin hydrochloride, and reacting at 100°C for 20 hours under stirring conditions, Obtain 5',8-bis(dimethylbiguanide base) luteolin hydrochloride, wherein n(metformin hydro...
Embodiment 2
[0039] Preparation of 5',8-bis(dimethylbiguanidino)luteolin-chromium(III) complex:
[0040] Step a: Dissolve NBS in acetone and stir at room temperature, avoid light, dissolve as much as possible, pour into the dropping funnel for dropwise addition, dissolve luteolin in acetone, add BPO, put the dropping funnel under ice bath Start to add slowly, and after the dropwise addition, heat to 70°C and react for 12 hours. After the reaction, carry out vacuum distillation to remove acetone, wash with deionized water several times and then freeze-dry to obtain bromoluteolin, wherein n(luteolin):n(NBS)=1:1.1, m(BPO):m(luteolin)=4%;
[0041] Step b: adding the bromoluteolin prepared in step a into a three-necked flask, using absolute ethanol as a solvent, sodium carbonate as a base, potassium iodide as a catalyst, adding metformin hydrochloride, and reacting at 95° C. and stirring for 18 hours, Obtain 5',8-bis(dimethylbiguanide) luteolin hydrochloride, wherein n(metformin hydrochloride)...
Embodiment 3
[0050] Preparation of 5',8-bis(dimethylbiguanidino)luteolin-chromium(III) complex:
[0051] Step a: Dissolve NBS in acetone and stir at room temperature, avoid light, dissolve as much as possible, pour into the dropping funnel for dropwise addition, dissolve luteolin in acetone, add BPO, put the dropping funnel under ice bath Start to add slowly, after the dropwise addition is completed, heat to 60°C, react for 9 hours, after the reaction, carry out vacuum distillation, evaporate acetone, wash with deionized water several times and freeze-dry to obtain bromoluteolin, wherein n(luteolin):n(NBS)=1:1.2, m(BPO):m(luteolin)=3%;
[0052] Step b: adding the bromoluteolin prepared in step a into a three-necked flask, using absolute ethanol as a solvent, sodium carbonate as a base, and potassium iodide as a catalyst, adding metformin hydrochloride, and reacting at 85° C. and stirring for 14 hours, Obtain 5',8-bis(dimethylbiguanide base) luteolin hydrochloride, wherein n(metformin hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com