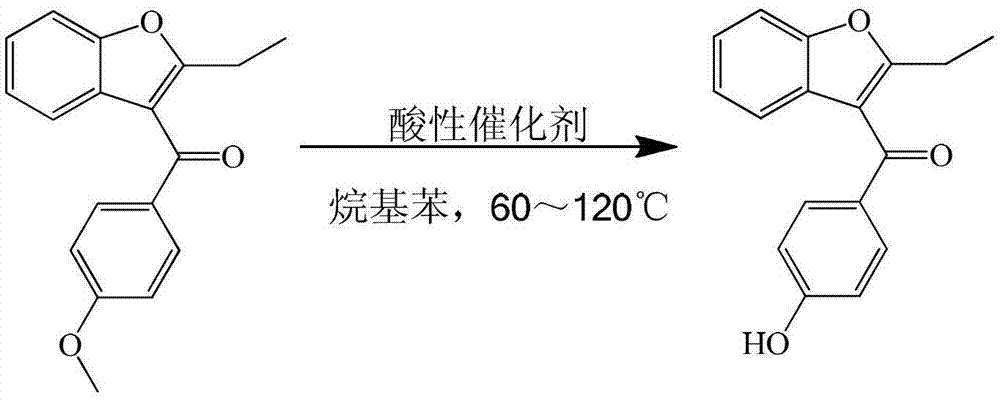
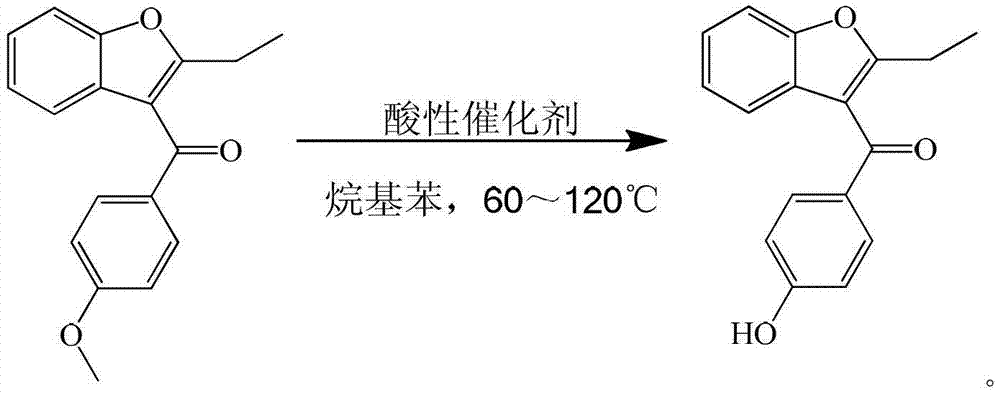
Process for preparing key intermediate 2-ethyl-3-p-hydroxybenzoyl-benzofuran of benzbromarone
A technology of hydroxybenzoyl and benzofuran, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as large fluctuations in yield, difficulty in purchasing, and hidden dangers in workshop safety production, and achieves mild reaction conditions, stable and reliable processes, and low price. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 250ml of xylene, 28g (0.1mol) of 2-ethyl-3-p-methoxybenzoyl-benzofuran, and 40.9g (0.3mol) of zinc chloride into a 500ml four-necked flask and stir at 60℃ for reaction 6 hour. After cooling with ice water, add 150ml of 25% dilute hydrochloric acid to separate the water layer. The organic layer is distilled under reduced pressure to recover the solvent. The organic layer is concentrated to dryness. Add 80ml of 15% sodium hydroxide solution to the concentrated organic layer to separate the water layer. Add 25% dilute hydrochloric acid to the water layer to adjust the pH=3, then add chloroform for extraction, and slowly distill the extract to dryness to obtain a solid, which is recrystallized and separated with petroleum ether to obtain 2-ethyl-3-pair Hydroxybenzoyl-benzofuran. The yield is 90%, and the liquid content is 99%.
Embodiment 2
[0021] Add 250ml of toluene, 28g (0.1mol) of 2-ethyl-3-p-methoxybenzoyl-benzofuran, 48.7g (0.3mol) of ferric chloride in a 500ml four-necked flask and stir at 95℃ for reaction 12 hour. After cooling with ice water, add 150ml of 25% dilute hydrochloric acid to separate the water layer. The organic layer is distilled under reduced pressure to recover the solvent. The organic layer is concentrated to dryness. Add 80ml of 15% sodium hydroxide solution to the concentrated organic layer to separate the water layer and the water layer. Add 25% dilute hydrochloric acid to adjust PH=3, then add chloroform for extraction, and slowly distill the extract to dryness to obtain a solid, which is recrystallized and separated with petroleum ether to obtain 2-ethyl-3-p-hydroxybenzene Formyl-benzofuran. The yield was 91%, and the liquid content was 98%.
Embodiment 3
[0023] Add 250ml of xylene, 28g (0.1mol) of 2-ethyl-3-p-methoxybenzoyl-benzofuran, and 56.9g (0.3mol) of titanium tetrachloride into a 500ml four-necked flask with stirring at 70℃. 6 hours. After cooling with ice water, add 150ml of 25% dilute hydrochloric acid to separate the water layer. The organic layer is distilled under reduced pressure to recover the solvent. The organic layer is concentrated to dryness. Add 80ml of 15% sodium hydroxide solution to the concentrated organic layer to separate the water layer and the water layer. Add 25% dilute hydrochloric acid to adjust the PH=3, then add chloroform for extraction, and slowly distill the extract to dry out to obtain a solid, which is recrystallized with petroleum ether to obtain 2-ethyl-3-p-hydroxyl Benzoyl-benzofuran. The yield is 90%, and the liquid content is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com