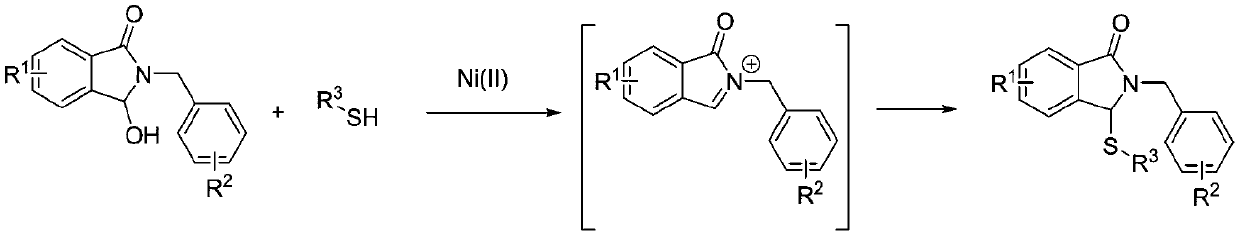
Synthesis method for 3-thioether isoindolinone compound
A technology for isoindolinone and ketone compounds, which is applied in the field of synthesis of 3-sulfide isoindolinone compounds, can solve problems such as expensive catalysts, complex catalyst structures, difficult synthesis reaction conditions, etc., to avoid The effect of expensive catalyst, simple raw materials and reagents, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
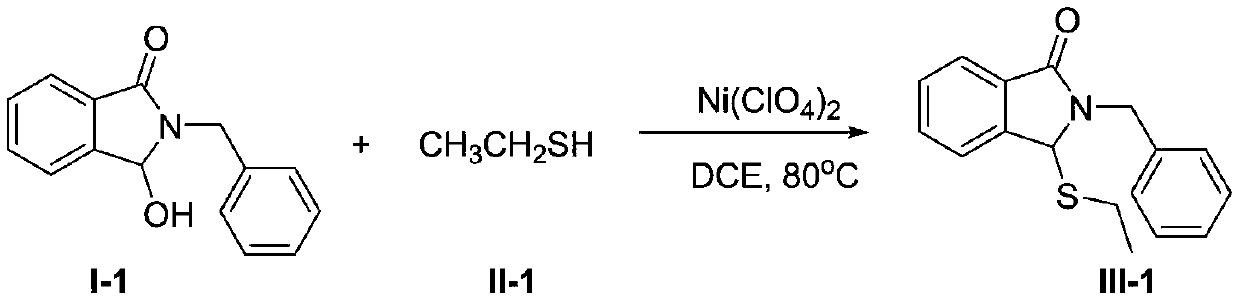
[0025] In the round bottom flask of 100mL, add 2.39g (10mmol) compound I-1, 1.24g (20mmol) compound II-1, 0.26g (1mmol) solid Ni(ClO 4 ) 2 , and finally 20 mL of dry 1,2-dichloroethane was added, and the resulting mixture was stirred at 80 °C for 10 hours. After the reaction mixture was cooled to room temperature, it was poured into ice water, extracted with 50mL×3 methylene chloride, and the extracted organic phases were combined, washed once with saturated brine, anhydrous Na 2 SO 4 Dry, filter, concentrate and remove the solvent to obtain a crude product, which is separated by column chromatography to obtain a pure product of compound III-1. Oily liquid, 2.60g, yield 92%. 1 HNMR (400MHz, CDCl 3 )δ: 7.87(d, J=7.5Hz, 1H), 7.57(s, 2H), 7.51–7.44(m, 1H), 7.30(dq, J=13.7Hz, 7.3Hz, 5H), 5.38(d, J=14.7Hz, 1H), 5.28(s, 1H), 4.39(d, J=14.7Hz, 1H), 1.96(dd, J=12.3Hz, 7.5Hz, 1H), 1.87–1.79(m, 1H) ,0.96(t,J=7.5Hz,3H); 13 C NMR (CDCl 3 ,100MHz)δ:167.33,143.66,136.91,...
Embodiment 2
[0027]
[0028] In the round bottom flask of 100mL, add 2.39g (10mmol) compound I-1, 1.52g (20mmol) compound II-2, 0.26g (1mmol) solid Ni(ClO 4 ) 2 , and finally 25 mL of dry DMF was added, and the resulting mixture was stirred at 100° C. for 5 hours until the reaction was complete. The reaction mixture was cooled to room temperature, poured into water, stirred, extracted with 50mL×3 dichloromethane, combined and extracted organic phases, washed once with saturated brine, anhydrous Na 2 SO 4 Dry, filter, concentrate and remove the solvent to obtain a crude product, which is separated by column chromatography to obtain a pure product of compound III-2. Oily liquid, 2.67g, yield 90%. 1 HNMR (400MHz, CDCl 3 )δ7.87(d, J=7.5Hz, 1H), 7.57(d, J=12.6Hz, 2H), 7.52–7.43(m, 1H), 7.29(ddd, J=22.2Hz, 13.8Hz, 6.9Hz ,5H),5.37(d,J=14.7Hz,1H),5.27(s,1H),4.39(d,J=14.7Hz,1H),1.90(dt,J=12.3Hz,7.4Hz,1H), 1.82–1.69(m,1H),1.28(dt,J=14.5Hz,7.2Hz,2H),0.80(t,J=7.3Hz,3H); 13 C NMR (CDCl 3 ,10...
Embodiment 3
[0030]
[0031] In the round bottom flask of 100mL, add 2.39g (10mmol) compound I-1, 1.35g (15mmol) compound II-3, 0.09g (0.5mmol) solid Ni(NO 3 ) 2 , and finally 50 mL of dry DMSO was added, and the resulting mixture was stirred vigorously at 120° C. for 5 hours. After the reaction mixture was cooled to room temperature, it was poured into water, stirred, extracted with 50mL×3 dichloromethane, combined and extracted organic phases were washed once with saturated brine, anhydrous Na 2 SO 4 Dry, filter, concentrate and distill off the solvent to obtain a crude product, which is purified by column chromatography to obtain a pure product of compound III-3. Oily liquid, 2.58g, yield 87%. 1 HNMR (400MHz, CDCl 3 )δ: 7.87(d, J=7.5Hz, 1H), 7.60–7.51(m, 2H), 7.51–7.44(m, 1H), 7.30(ddd, J=22.5Hz, 13.9Hz, 7.0Hz, 5H) ,5.37(d,J=14.7Hz,1H),5.28(s,1H),4.37(d,J=14.7Hz,1H),1.80(dd,J=12.0Hz,6.7Hz,1H),1.63(dd ,J=11.9Hz,6.9Hz,1H),1.46(dt,J=13.3Hz,6.7Hz,1H),0.81(t,J=6.2Hz,6H); 13 C NMR (CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com