Preparation and application of organoboron phosphorescent material with ultra-long lasting luminescent performance
A technology of luminescent properties and phosphorescent materials, applied in the field of preparation of organoboron phosphorescent materials, can solve the problems of low luminescent quantum efficiency, oxygen collision quenching, short life, etc., and achieve high luminescent quantum efficiency, simple synthesis method and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
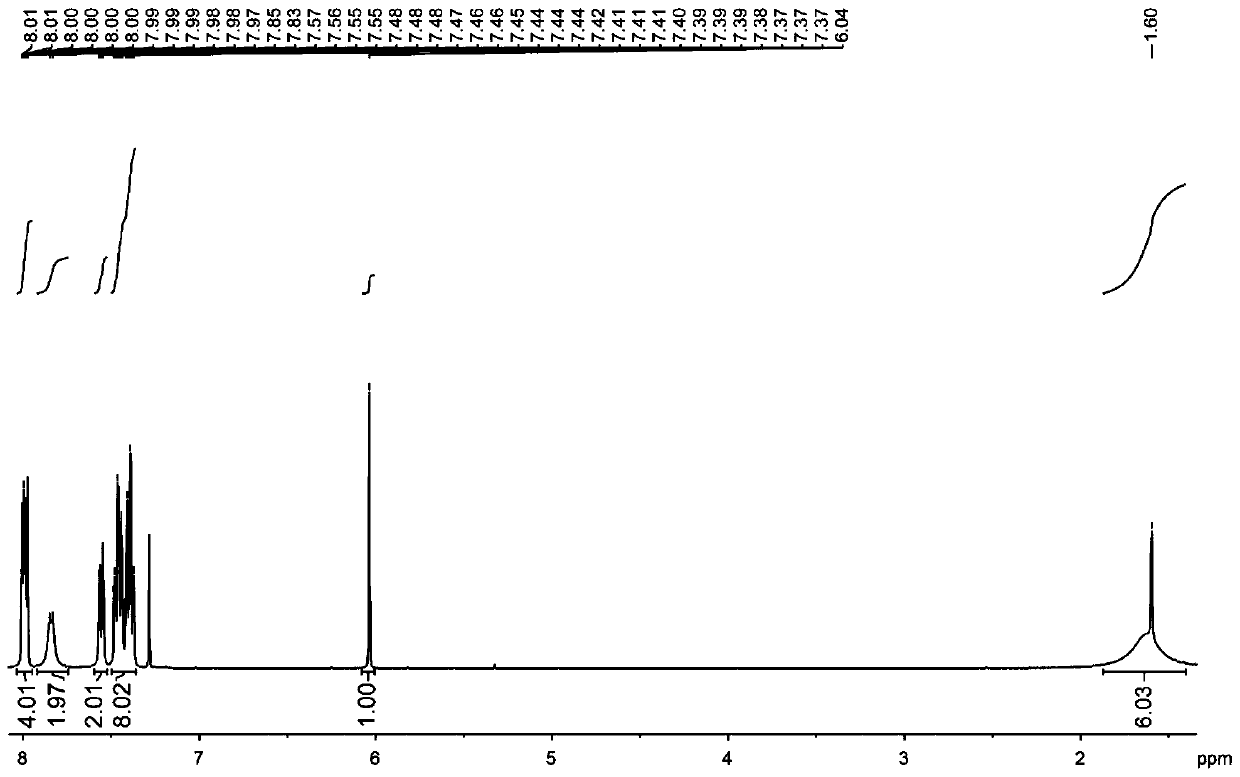
[0030] The structural formula of the target product: .
[0031] Synthesis of organoboron phosphorescent materials:
[0032] Step 1. Weigh 1.00 g of carbazole and 1.25 g of 9,9-diphenylacridine into a single-necked bottle, vacuum three times with nitrogen blowing, nitrogen protection, add 20 mL of freshly steamed toluene solution and stir to dissolve.
[0033] Step 2: Vacuum the one-necked bottle with nitrogen three times, under the protection of nitrogen, dissolve 0.58 mL of malonyl chloride in 10 mL of freshly steamed toluene solution, stir for 5 minutes, then add the reaction system in step 1 dropwise into the malonyl chloride toluene solution, stirred for two hours, extracted with water and dichloromethane, collected the organic phase, added silica gel powder and spin-dried, and purified by column chromatography to obtain the intermediate product, the eluent used was dichloromethane:petroleum Ether is 1:4.
[0034] Step 3, dry the intermediate product, vacuumize and blo...
Embodiment 2
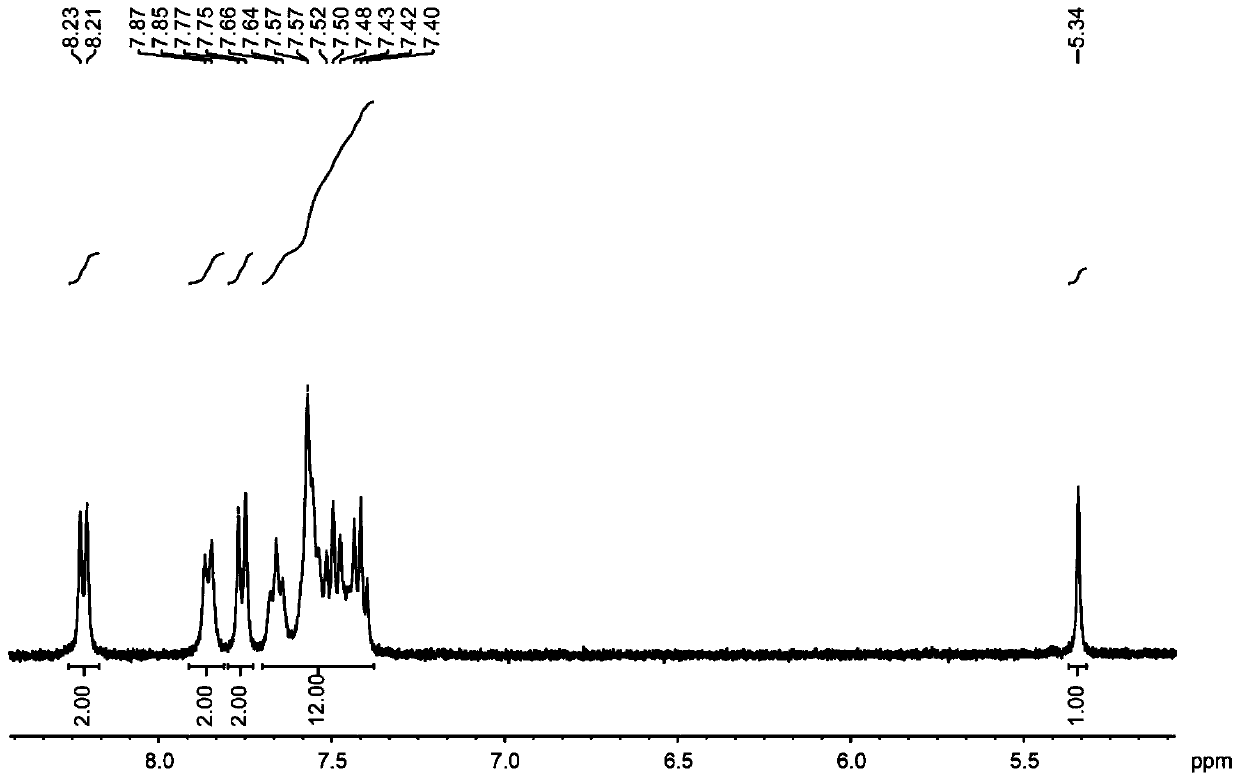
[0036] The structural formula of the target product: .
[0037] Synthesis of organoboron phosphorescent materials:
[0038] Step 1. Weigh 1.00 g of carbazole and 1.02 g of diphenylamine into a single-necked bottle, vacuum three times to blow nitrogen, and protect with nitrogen, add 20 mL of freshly steamed toluene solution and stir to dissolve.
[0039] Step 2: Vacuum the one-necked bottle with nitrogen three times, under the protection of nitrogen, dissolve 0.58 mL of malonyl chloride in 10 mL of freshly steamed toluene solution, stir for 5 minutes, then add the reaction system in step 1 dropwise into the malonyl chloride toluene solution, stirred for two hours, extracted with water and dichloromethane, collected the organic phase, added silica gel powder and spin-dried, and purified by column chromatography to obtain the intermediate product, the eluent used was dichloromethane:petroleum Ether is 1:4.
[0040] Step 3: Dry the intermediate product, vacuumize and blow nitr...
Embodiment 3
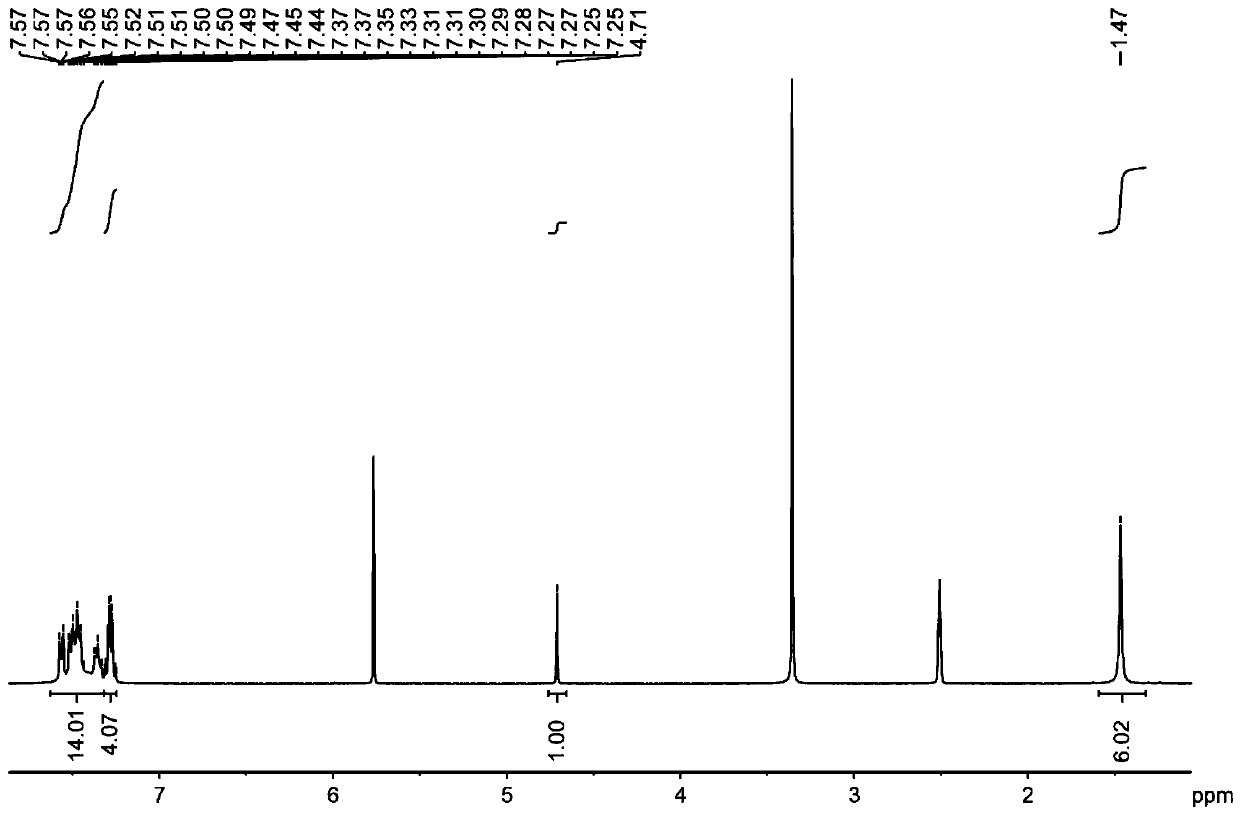
[0042] The structural formula of the target product: .
[0043] Synthesis of organoboron phosphorescent materials:
[0044] Step 1. Weigh 1.25 g of 9,9-diphenylacridine and 1.02 g of diphenylamine into a single-necked bottle, vacuum three times with nitrogen blowing, nitrogen protection, add 20 mL of freshly steamed toluene solution and stir to dissolve.
[0045] Step 2: Vacuum the one-necked bottle with nitrogen three times, under the protection of nitrogen, dissolve 0.58 mL of malonyl chloride in 10 mL of freshly steamed toluene solution, stir for 5 minutes, then add the reaction system in step 1 dropwise into the malonyl chloride toluene solution, stirred for two hours, extracted with water and dichloromethane, collected the organic phase, added silica gel powder and spin-dried, and purified by column chromatography to obtain the intermediate product, the eluent used was dichloromethane:petroleum Ether is 1:4.
[0046] Step 3: Dry the intermediate product, vacuumize and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com