Arylamine compound and organic light-emitting device thereof
A technology of organic light-emitting devices and compounds, which is applied in the field of organic photoelectric materials, can solve problems such as the reduction of light-emitting area, and achieve the effects of high refractive index, compact electron stacking, and strong photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0152] The preparation and formation methods of each layer in the organic light-emitting device are not particularly limited, and vacuum evaporation method, spin coating method, vapor deposition method, blade coating method, laser thermal transfer method, electrospray coating method, slit coating method can be used. Either of the cloth method and the dip coating method, the method of vacuum vapor deposition is preferably used in the present invention.
[0153] The organic light-emitting device of the present invention can be widely used in the fields of panel display, lighting source, flexible OLED, electronic paper, organic solar cell, organic photoreceptor or organic thin film transistor, signboard, signal lamp and the like.
Embodiment 1
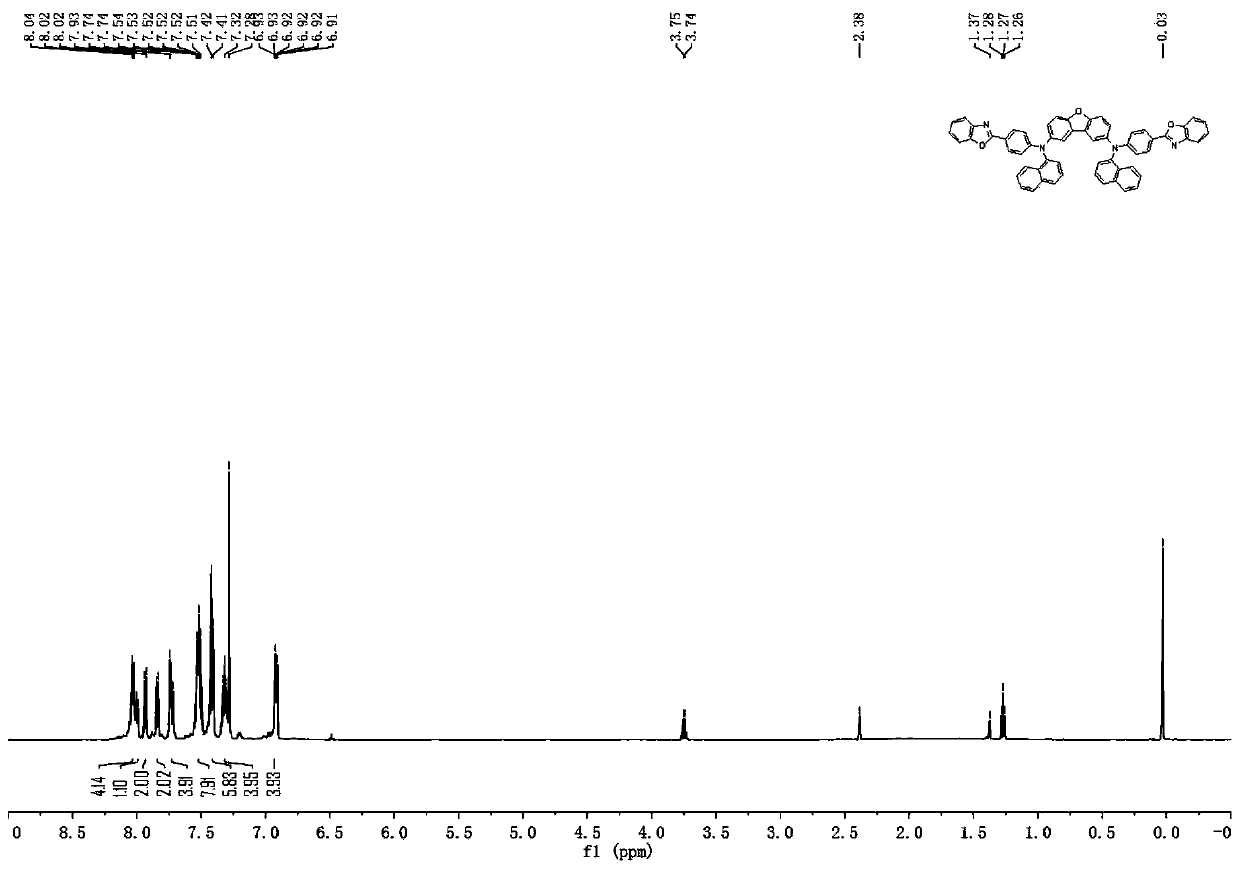
[0161] [Example 1] Synthesis of Compound 4
[0162]
[0163] Synthesis of Intermediate I-4
[0164] Under nitrogen protection, toluene (600mL), 4-(2-benzoxazolyl)aniline (44.15g, 0.21mol), 1-brominated naphthalene (43.48g, 0.21mol), Palladium acetate (0.61 g, 0.0027 mol), sodium tert-butoxide (33.7 g, 0.351 mol) and tri-tert-butylphosphine (10.8 mL of a 1.0 M solution in toluene, 0.0108 mol). And react under the condition of reflux for 2 hours. After the reaction stopped, the mixture was cooled to room temperature, filtered with diatomaceous earth, the filtrate was concentrated, recrystallized with methanol, filtered with suction and rinsed with methanol to obtain a recrystallized solid to obtain intermediate I-4 (61.45 g, the yield was about 87 %), HPLC detection solid purity >= 98.1%.
[0165] Synthesis of Compound 4
[0166] Add toluene solvent (450ml), 2,8-dibromodibenzofuran (11.74g, 36mmol), intermediate I-4 (30.27g, 90mmol), Pd 2 (dba) 3 (990mg, 1.08mmol), BINA...
Embodiment 2
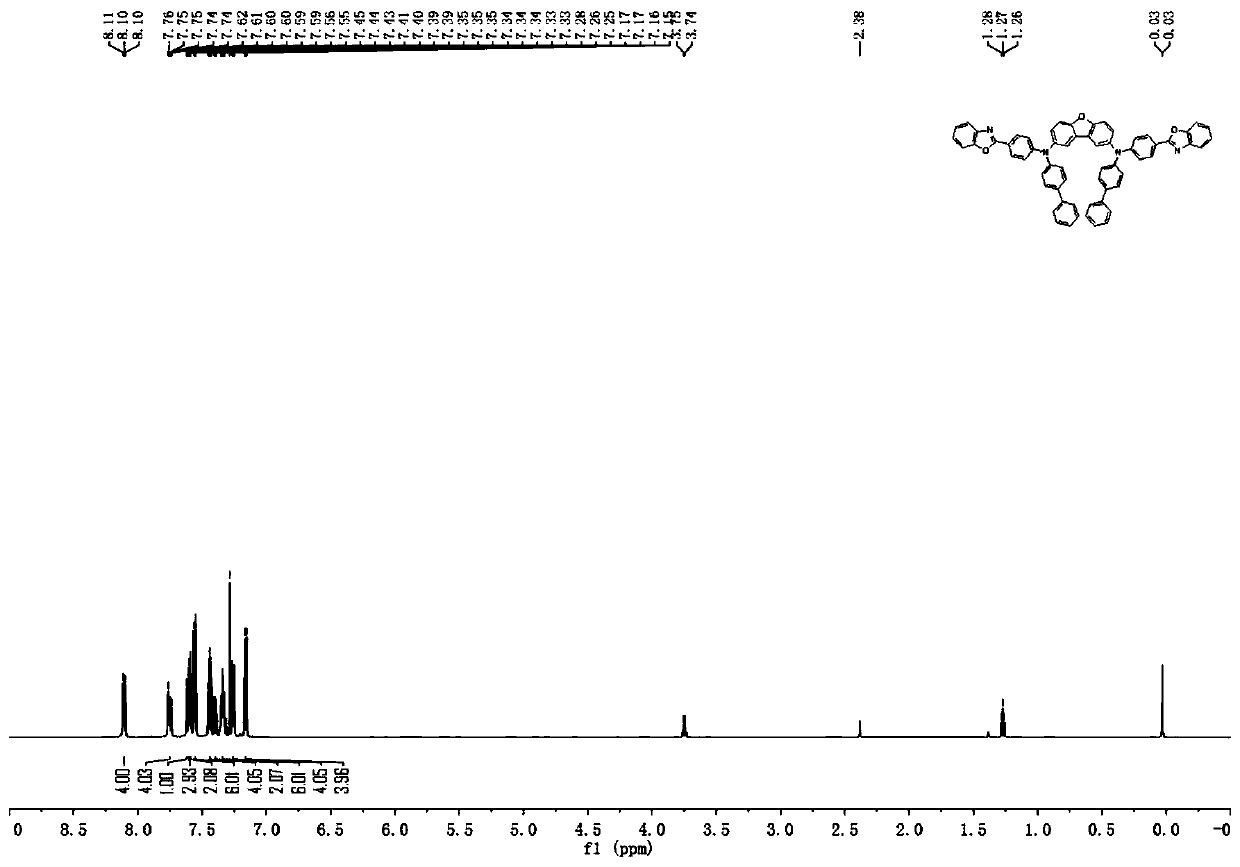
[0168] [Example 2] Synthesis of compound 11
[0169]
[0170] Synthesis of Intermediate I-11
[0171] Under the protection of nitrogen, add toluene (600mL), 4-(2-benzoxazolyl)aniline (44.15g, 0.21mol), 4-bromobiphenyl (48.95g, 0.21mol) successively into the 1L reaction flask , palladium acetate (0.61 g, 0.0027 mol), sodium tert-butoxide (33.7 g, 0.351 mol) and tri-tert-butylphosphine (10.8 mL of a 1.0 M solution in toluene, 0.0108 mol). And react under the condition of reflux for 2 hours. After the reaction stopped, the mixture was cooled to room temperature, filtered with diatomaceous earth, the filtrate was concentrated, recrystallized with methanol, filtered with suction and rinsed with methanol to obtain a recrystallized solid to obtain intermediate I-11 (66.97g, the yield was about 88 %), HPLC detection solid purity >= 98.0%.
[0172] Synthesis of compound 11
[0173] Add toluene solvent (450ml), 2,8-dibromodibenzofuran (11.74g, 36mmol), intermediate I-11 (32.62g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com