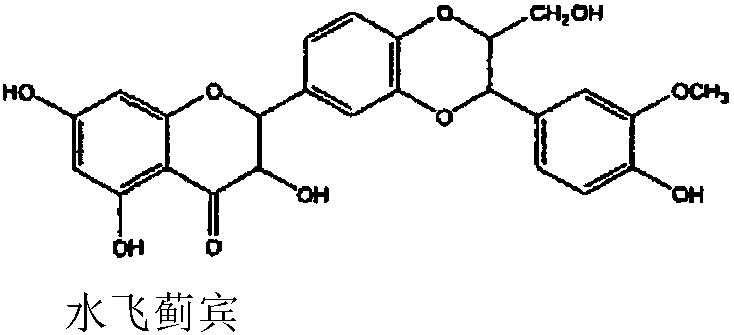
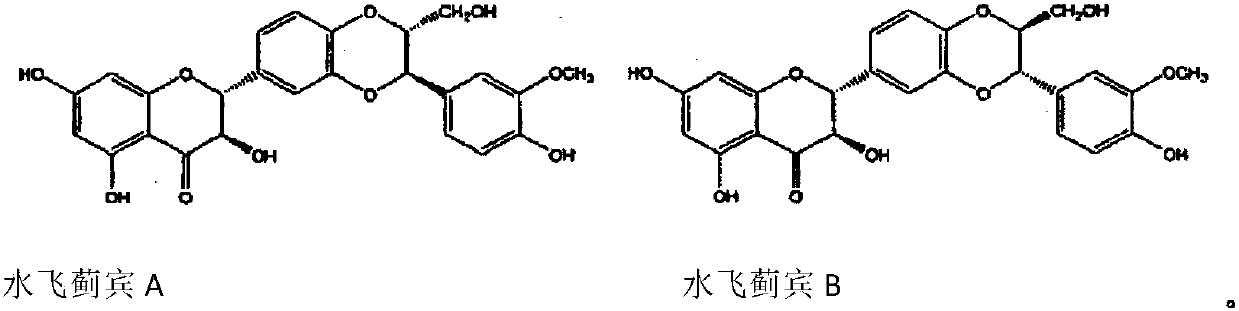
Pharmaceutical use of flavanonol natural products
A drug, the technology of silibinin, which is applied in the field of medicinal use of natural products of dihydroflavonols, can solve the problems such as the elaboration of the therapeutic effect of NASH
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] 1 Grouping of experimental animals
[0021] After single-cage adaptation and gavage adaptation, the animals were divided into 9 groups according to their body weight and food consumption. The mice were adapted for one week, and the mice in groups 2-8 began to be fed with MCD feed. Co-feeding induced 6 weeks, while the normal group Feed mice with normal chow.
[0022] 2 Construction of NASH animal model
[0023] The NASH model induced by a methionine-choline-deficient (MCD) diet was adopted, and MCD is an animal model widely recognized internationally.
[0024] 8-week-old male mice, weighing 18-22g. The body weight of the animals was weighed before the start of MCD modeling. Two weeks after modeling, blood was taken to test serum TG, TC, ALT and AST, and liver was taken to test TG and TC. The NAS pathological score of the model group was greater than 5 points, indicating that the NASH model was successfully constructed. The mice were fed with MCD for 6 weeks.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com