Novel multi-specific binding proteins
A technology for binding proteins and binding regions, which can be used in hybrid immunoglobulins, immunoglobulins, anti-growth factor immunoglobulins, etc., and can solve problems such as low activity, indistinguishability, and stability problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1. Structure of a new bispecific antibody format (Fv2mab)
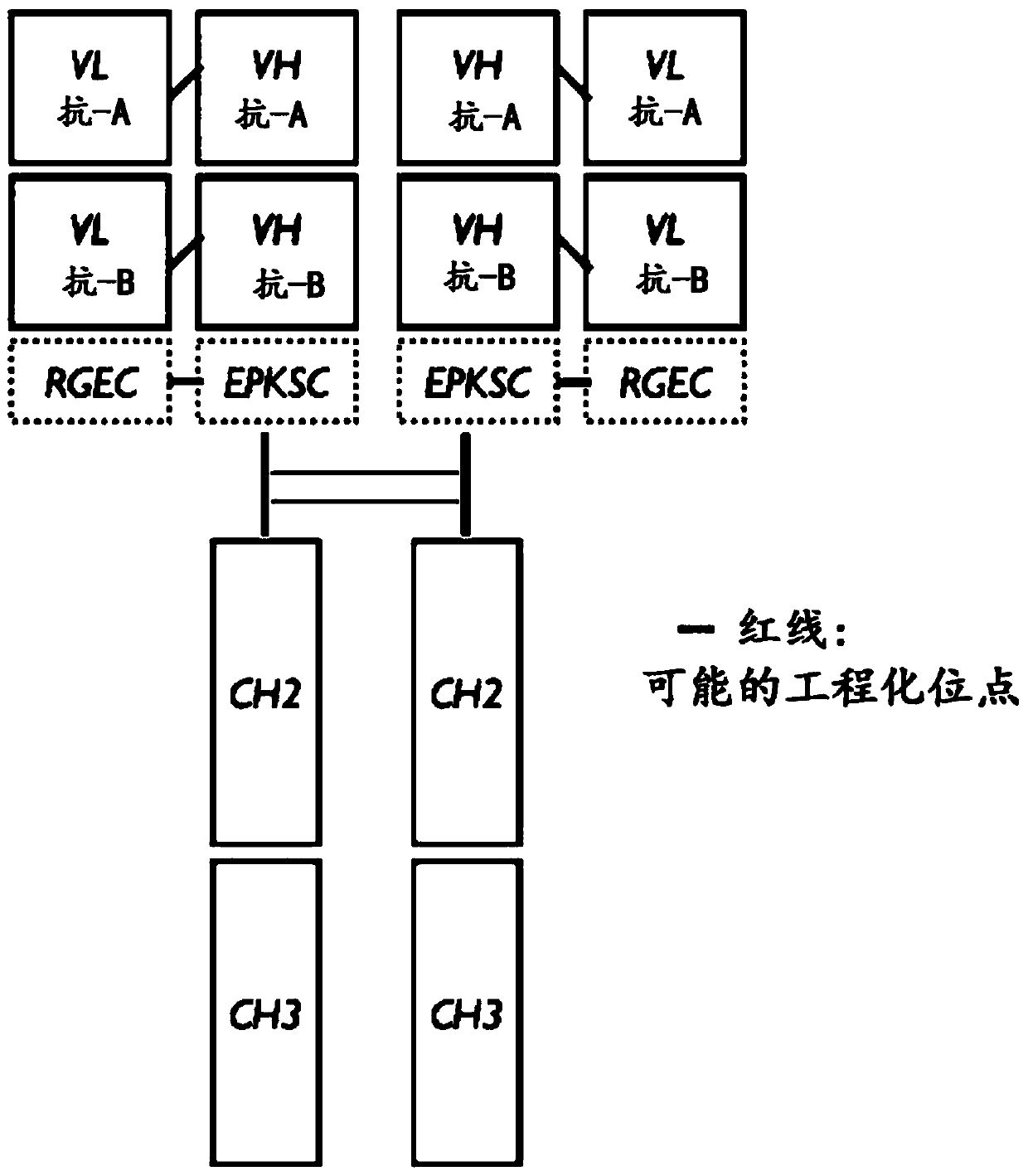
[0103] Fv2mab is a bispecific antibody with a variable domain (Fv ) The tandem structure of fragments. Two or more kinds of variable regions connected in different series have the ability to bind different antigens independently or simultaneously, and have a form in which the heavy chain CH1 domain, kappa or lambda and light chain CL domain are removed ( Figure 1a ).
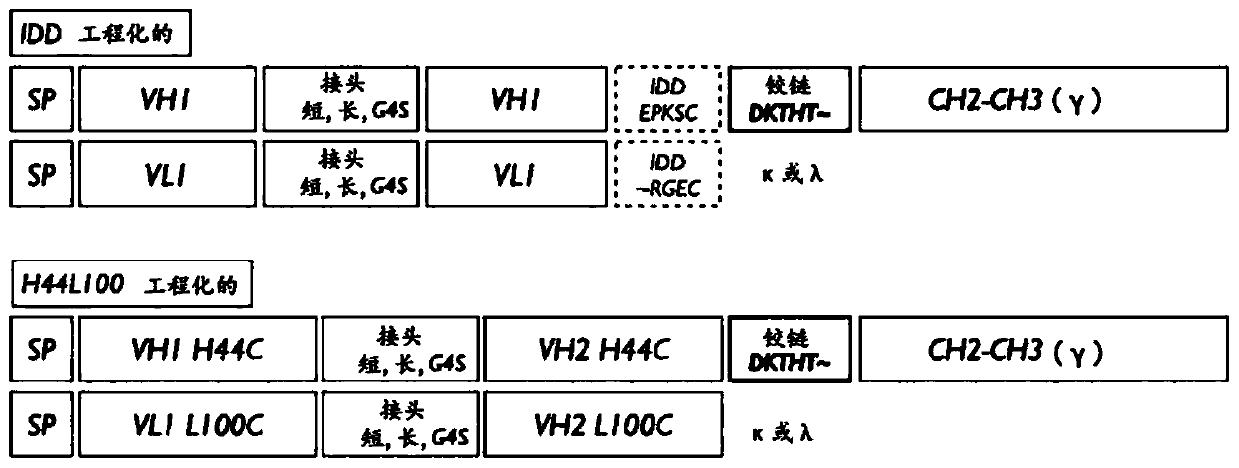
[0104] To guarantee binding affinity for a single antigen corresponding to two tandem-linked variable domains (Fv), the short linker (VH: ASTKGP SEQ; ID NO: 1, VL: TVAAP; SEQ ID NO: 4), the long linker (VH: ASTKGPSVFPLAP; SEQ ID NO: 2, VL: TVAAPSVFIFPP; SEQ ID NO: 5) and G4S linker (VH: GGGGSGGGGS; SEQ ID NO: 3, VL: GGSGGGGSG; SEQ ID NO: 6) for external variable structures Domains and internal variable domains to ensure the structural independence of each variable domain ( figure 2 ).
[0105] Parental antibodies used as proof of co...
Embodiment 2
[0106] Example 2. Fv2mab Engineering for Thermodynamic Stability
[0107] The structure of the Fv2mab bispecific antibody is H with two or more consecutive Fv (VH and VL) 2 L 2 multivalent tetramer. In order to tetramer (H 2 L 2 ) structure exists, and the CH domain of the heavy chain and the CL domain of the light chain of the universal antibody are connected by an interchain disulfide bridge (covalent bond). However, in the case of Fv2mabs lacking CH1 and CL, the structure and stability are governed by the binding forces (hydrophobic, ionic, hydrogen-bonded, etc.), and in contrast to universal antibodies, there are no intrachain disulfide bridges (covalent bonds) between the heavy and light chains. Therefore, the binding force to determine the structure of the heavy and light chains at low concentrations is expected to be lower than that of a generic antibody, and therefore, the pK / pD is also expected to be lower than in the case of a generic antibody.
[0108] Therefo...
Embodiment 3
[0112] The preparation of embodiment 3.Fv2mab
[0113] By using Humira (adalimumab, AbbVie), KOSENTYX (secukinumab, Novartis), anti-VEGFA (in-house antibody) and ABT-122 (DVD-Ig, AbbVie) heavy chain variable region and light chain Sequences of variable regions Proof-of-concept (POC) parental antibodies were prepared for various combinations of bispecific Fv2 mabs. The pFE expression vector prepared by the applicant is used as an animal cell expression vector for the production of the recombinant protein of the bispecific antibody Fv2mab. The heavy and light chain variable regions of antibodies consist of hypervariable regions (CDRs) and frameworks (FRs). Since FR1 as the starting point and FR4 as the ending point in the variable region consist of similar amino acid sequences in most antibodies, it is difficult to connect two kinds of variable regions in tandem by a method such as PCR. This is because it is difficult to prepare and use specific binding primers due to the simi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com