Method for manufacturing hyaluronate film, and hyaluronate film manufactured thereby
A technology of hyaluronate and film, which is applied in the fields of flat products, applications, medical science, etc. It can solve the problems that the research of hyaluronate film is still blank, and the toxic cross-linking agent has not been completely removed, so as to achieve easy mass production , easy physical properties, easy to adjust the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of hyaluronate film using solvent casting
[0065] 1-1: Confirmation of film forming ability according to molecular weight change
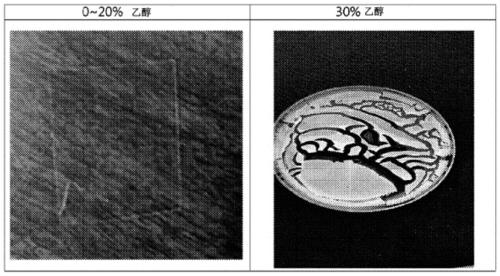
[0066] In order to confirm the film-forming ability according to the molecular weight of hyaluronate, a total of four kinds of sodium hyaluronate (Hi-Aqua TM ), (Jinwoo Bio)) were dissolved in 20 volume percent ethanol aqueous solution with a concentration of 0.5 to 10.0% by weight, and they were injected into acrylic resin molds respectively, and then dried in a constant temperature and humidity dryer (relative humidity 50 %, temperature 40° C.) for 6 to 24 hours to prepare a hyaluronate film.
[0067] As a result, it was confirmed that in the case of molecular weights of 0.8, 1.2, and 2.5 MDa, when the concentration was 0.5% or more, a film was formed well, but when 0.1 MDa was dissolved at 5% or more, a film was formed well.
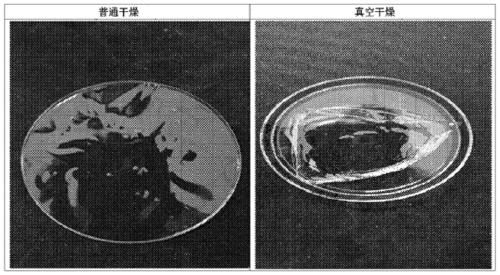
[0068] As a result of measuring the moisture content of the prepared hyaluronate film u...
experiment example 1
[0082] Experimental Example 1: Measurement of mechanical properties of hyaluronate film
[0083] In 20 volume percent ethanol aqueous solution, sodium hyaluronate (hyaluronate (Hi-Aqua) with a molecular weight of 0.8MDa, 1.2MDa and 2.5MDa TM ), (strain) Zhenyou Biological) dissolved with a concentration of 1.0% by weight, and the molecular weight is 0.1MDa hyaluronic acid, dissolved with a concentration of 5% by weight, injected into acrylic resin molds respectively, and then dried in a constant temperature and humidity dryer (relative humidity 50%) , temperature 40° C.) for 12 hours to prepare a hyaluronate film.
[0084] In order to measure the mechanical properties of the prepared hyaluronate film, use TA-XT2i Texture Analyzer (texture analyzer) (Stable Micro System (Stable Micro System), UK), cut the prepared film into 3cm × 5cm, add After the tensile grips (Tensile grips) part, the tensile strength and tensile ratio were measured, and the results are shown in Table 1.
...
experiment example 2
[0088] Experimental example 2: Stability measurement of hyaluronate film against microorganisms
[0089] The total number of bacteria refers to the number of bacteria that can develop in the standard agar medium among the bacteria present in the sample. In order to evaluate the contamination stability of the hyaluronate film to microorganisms, the sample and the standard agar medium were mixed and solidified in a petri dish, and the number of viable bacteria in the sample was calculated from the number of bacterial colonies that occurred after cultivation.
[0090] Preparation of test solution and reagent
[0091] -Standard agar medium (Plate count agar)
[0092] Distilled water was added to 5.0 g of tryptone, 2.5 g of yeast extract, 1.0 g of dextrose, and 15.0 g of agar to make 1,000 mL, adjusted to pH 7.0±0,2, and then sterilized at 121°C for 15 minutes.
[0093] sterile saline
[0094] Sterilized with 0.9% sodium chloride aqueous solution at 121°C for 15 minutes before u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com