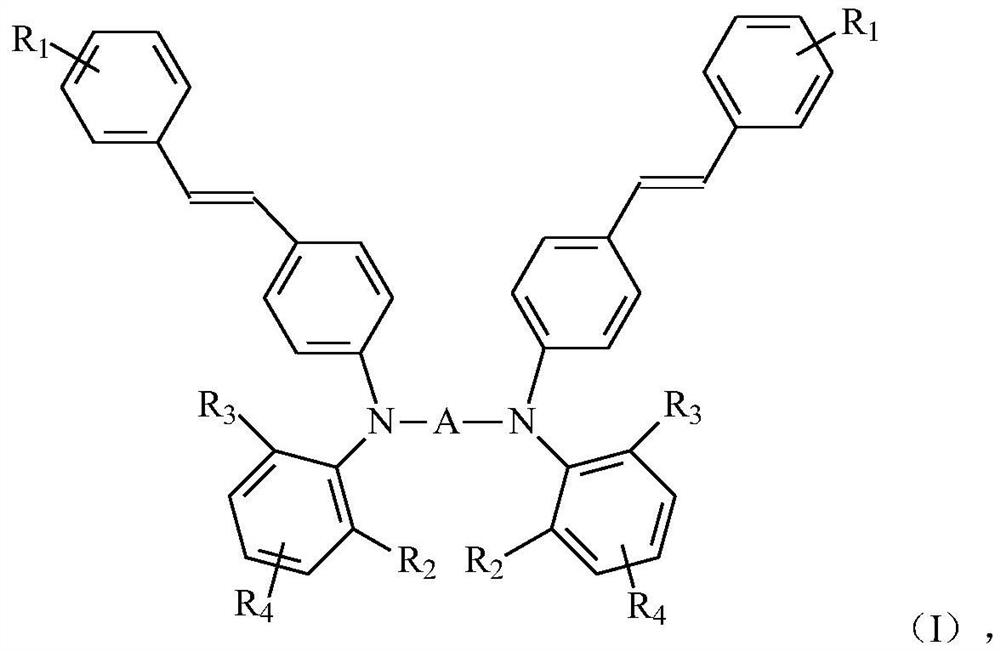
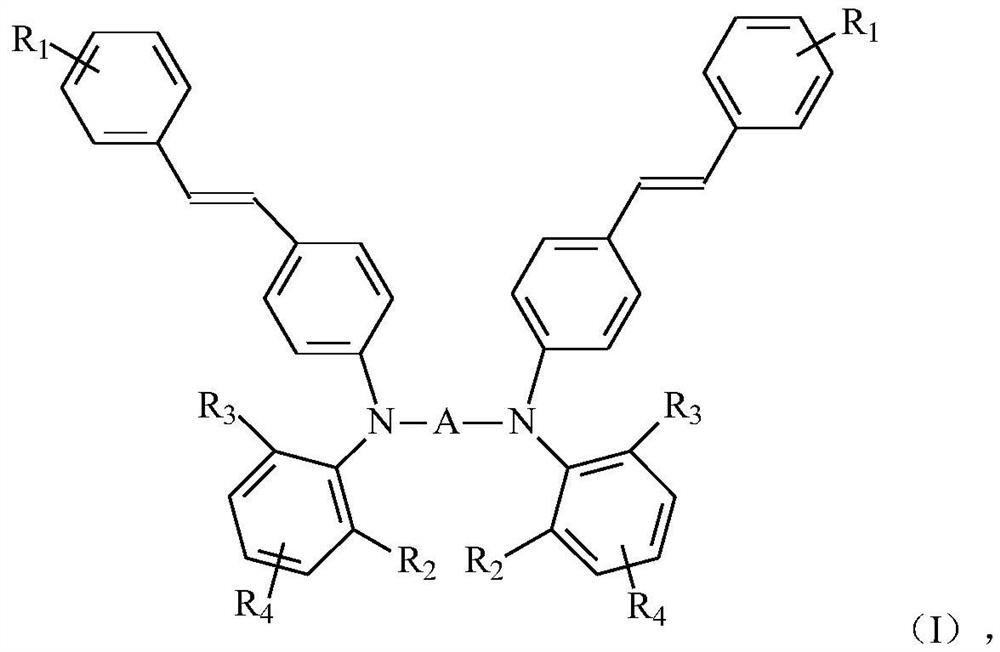
Tertiary amine photosensitizer, preparation method thereof, photosensitive resin composition containing same and application of photosensitive resin composition
A technology of photosensitive resin and tertiary amine photosensitizer, applied in the field of tertiary amine photosensitizer, photosensitive resin composition, and photosensitive resin composition, can solve problems such as low solubility and sensitizer precipitation, and achieve sensitive High compatibility, good compatibility, stable production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
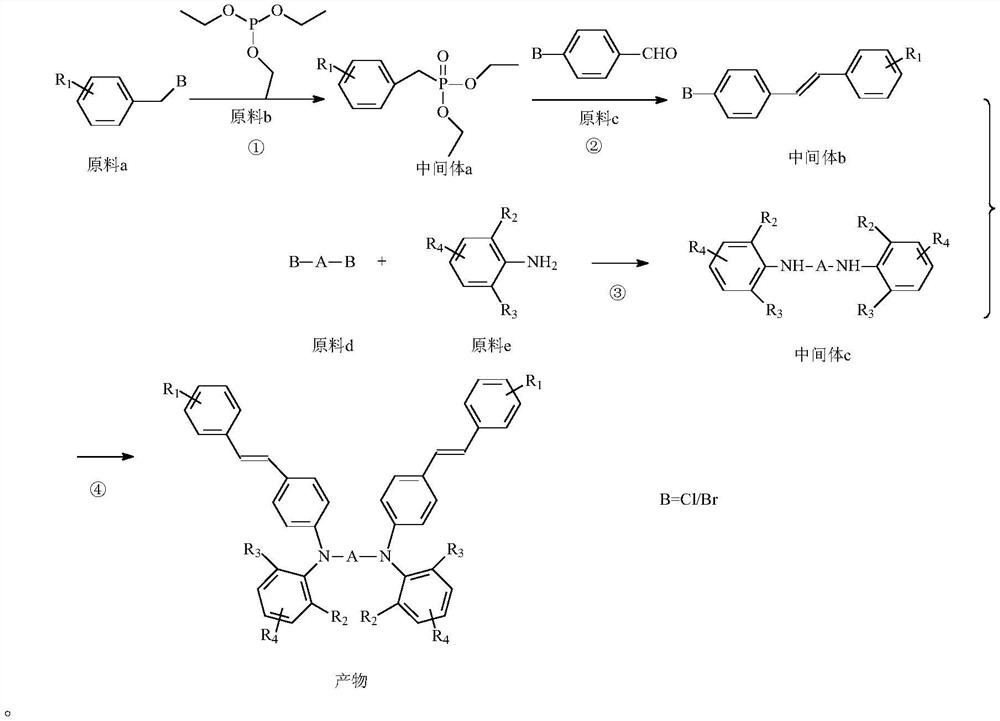
[0083] According to a typical embodiment of the present invention, a preparation method of the above-mentioned tertiary amine photosensitizer is provided. The preparation method comprises the following steps: 1) react raw material a and raw material b to obtain intermediate a; 2) react intermediate a and raw material c in an organic solvent containing a catalyst to obtain intermediate b; 3) react raw material d and raw material e in nitrogen Under the protection of the catalyst, the intermediate c is obtained; (4) the intermediate b and the intermediate c are obtained through the catalytic reaction of the catalyst under the protection of nitrogen to obtain the product; the reaction equation is as follows:
[0084]
[0085] In the case of knowing the above reaction scheme, the specific reaction conditions in steps 1) to 4) can be easily determined by those skilled in the art.
[0086] The reaction of step 1) can be carried out under catalyst-free and solvent-free conditions....
Embodiment 1
[0098] Component D is the preparation of the tertiary amine derivative shown in general formula (I)
[0099] (1) Preparation of intermediate a1
[0100]
[0101] Add 63 g of benzyl chloride and 100 g of triethyl phosphite into a 500 ml four-neck flask, stir and raise the temperature to 80-90° C., and react for 3 h. GC (gas chromatography) controls to benzyl chloride less than 1%, and the reaction ends. Distill at 90°C atmospheric pressure to remove the reaction by-product ethyl chloride, continue to distill under reduced pressure to remove unreacted triethyl phosphite, after no fraction is removed, cool down to 30°C to obtain 105g of intermediate a1 with a yield of 92.1% .
[0102] The structure of intermediate a1 via 1 H-NMR and LCMS confirmed.
[0103] 1 H-NMR (CDCl 3 , 500MHz): δ1.11 (6H, m), δ3.57 (4H, s), δ5.33 (2H, s), δ7.06-7.14 (5H, m).
[0104] MS (m / z): 229 (M+1).
[0105] (2) Preparation of intermediate b1
[0106]
[0107] Add 114g of intermediate a1...
Embodiment 2
[0124] (1) Preparation of intermediate c2
[0125]
[0126] Add raw materials 162.5g d2, 2-ethyl-6-methylaniline 216g, toluene 400g in a 1000mL four-necked flask, add sodium tert-butoxide 40g, tri-tert-butylphosphine 36g and tetrakis(triphenyl Phosphine)palladium 1.6g, heat up to 80-85°C and keep it warm for 10h, and control in HPLC until the reaction of raw material d2 and its intermediate monosubstituted product is complete (HPLC: <0.1%). Filtrate while hot, distill off the solvent from the mother liquor under reduced pressure, add 200 g of n-hexane, cool to 10° C., stir and crystallize, and filter with suction to obtain 197.1 g of intermediate c2 with a purity of 91%.
[0127] The structure of product 1 was determined by LCMS and 1 H-NMR confirmed.
[0128] 1 H-NMR (CDCl 3 , 500MHz): δ1.24-2.59 (16H, m), δ4.01 (2H, s), δ6.28-7.15 (12H, m), δ10.1 (1H, s).
[0129] MS (m / z): 434 (M+1).
[0130] (2) Preparation of product 2
[0131]
[0132] Add 208g of intermedia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com