Continuous synthesis method and device of amion-2,2,6,6-tetramentylniperidine
A technology for the synthesis of tetramethylpiperidinamine, which is applied in the field of synthesis of tetramethylpiperidinamine, can solve the problems of increasing the post-treatment process of organic solvents, the large proportion of organic solvents, and complicated operation processes, so as to shorten the production cycle , Reaction efficiency improvement, high mixing efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
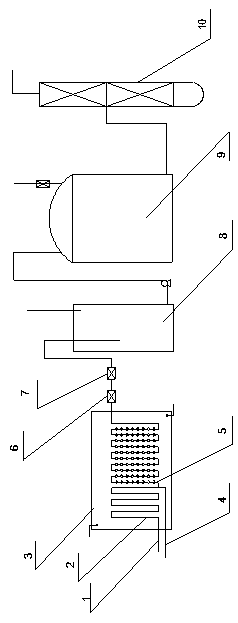
[0018] Embodiment 1: see figure 1 , a continuous synthetic method of tetramethylpiperidinamine, the synthetic method comprises the following steps: taking triacetone amine as raw material, adding a catalyst, feeding ammonia gas, reacting in the first channel 2 of the microchannel, the temperature is 50~ 150°C, time 5~300s, pressure 0.3~3Mpa, pass hydrogen gas into the second channel 5 of the microchannel, the temperature of hydrogen intake is 50~150°C, time 10~300s, pressure 0.3~3Mpa, the obtained reaction liquid passes through the gas After the liquid is separated, it enters the rectification tower for rectification to obtain the finished product. In this solution, preferably, the temperature in the first channel is 50-80°C, the time is 10-60s, the pressure is 0.5-2Mpa, the temperature in the second channel is 70-120°C, the time is 20-150s, Pressure 0.5~2Mpa. The method adopts a microchannel reactor, which occupies a small area and has few potential safety hazards. In addit...
Embodiment 2
[0019] Example 2: see figure 1 , a tetramethylpiperidinamine continuous synthesis device, the synthesis device includes a microchannel reactor 3, a gas-liquid splitter 8, a storage tank 9 and a rectification tower 10, the microchannel reactor is provided with micro Channel first channel 2 and microchannel second channel 5, a pressure reducing valve 6 and a throttle valve 7 are arranged between the microchannel reactor 3 and the gas-liquid separator 8, and the storage tank 9 is arranged on the gas-liquid separator Between the gas-liquid separator and the rectification tower, there is an online hydrogen detector and a liquid level linkage pump at the outlet of the gas-liquid separator. The first channel of the microchannel is a straight micropore to completely react the triacetone amine and ammonia. The aperture is 0.1~20mm, and the tube length is 10~100m. Preferably, the pore diameter of the first channel is 0.5-10mm, the tube length is 20-60m, and the second channel of the mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com