Preparation method for dendritic cell (DC) tumor vaccine
A technology for dendritic cells and tumor vaccines, which is applied in the field of preparation of dendritic cell tumor vaccines, can solve problems such as difficulty in achieving expected effects, and achieve the effects of low cost, promotion of endocytosis of tumor antigens, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 (preparation of tumor vaccine)
[0021] The tumor vaccine described in this example consists of the following steps:
[0022] 1. Add the mouse-derived liver cancer cell body into the DMEM (or RPMI-1640) culture solution containing serum, then add the culture solution with a platycodon saponin D concentration of 5uM, and place it in an incubator with a carbon dioxide concentration of 5%. Incubate at 37°C for more than 2 hours. The purpose of this step is to reduce the expression of sialic acid in tumor cells without causing death of tumor cells, so that antigens covered by sialic acid on the surface of tumor cells can be exposed and recognized by dendritic cells.
[0023] 2. Digest the tumor cells cultured in step (1) with trypsin, then add dendritic cells in the ratio of tumor cell number: dendritic cell number = 0.5: 1 for co-cultivation for more than 2 hours, and add lipopolysaccharide at a concentration of 100 ~300ng / ml of LPS was continued to culture,...
Embodiment 2
[0027] Example 2 (An experiment in which platycodon saponin D inhibits sialic acid in murine breast cancer cells and enhances the effect of dendritic vaccines in preventing breast cancer)
[0028] (1) The effect of the traditional Chinese medicine platycodon saponin D on five highly expressed sialyltransferase genes in breast cancer cells
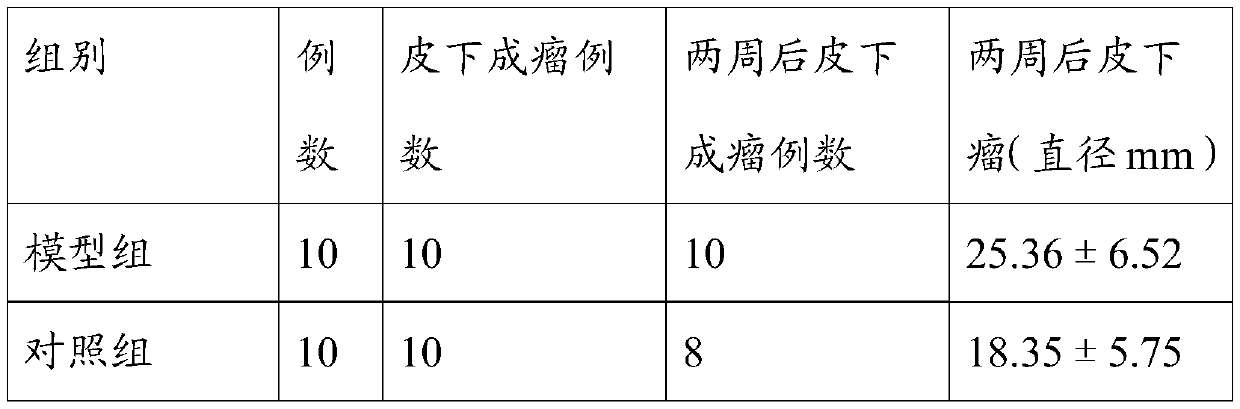
[0029] The experiments were divided into tumor model group and 5μM Platycodon grandiflora group.
[0030] Breast cancer cells were inoculated into 6cm culture dishes, 5mL per dish, and then cultured in an incubator. Add 5 mL of complete culture solution to the blank control group, add 5 mL of 5 μM PD drug-containing culture solution to the experimental group, and place them in an incubator to continue culturing for 48 hours. When the cells of the two groups grew to a density of 80%, the cells were collected and the total RNA of the cells was extracted. cDNA was reverse transcribed using a reverse transcription kit.
[0031] The cDNA obta...
Embodiment 3
[0047] Example 3 (An experiment of platycodon saponin D inhibiting sialic acid in murine colon cancer cells and enhancing the effect of dendritic vaccines on colon cancer prevention)
[0048] (1) The effect of the traditional Chinese medicine platycodon saponin D on the highly expressed sialyltransferase gene in colon cancer cells
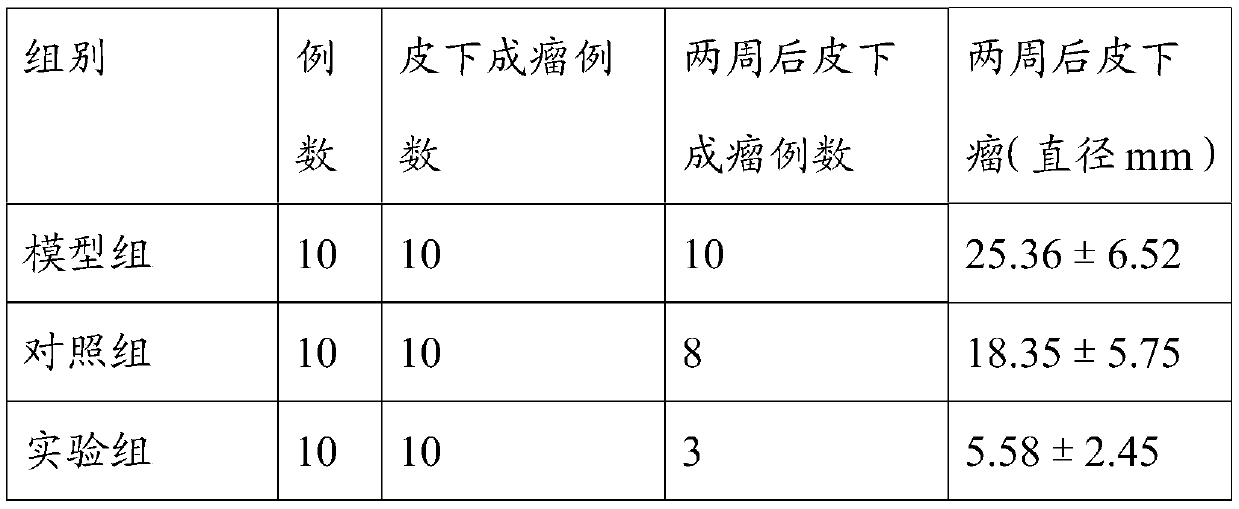
[0049] The experiments were divided into tumor model group and 10μM Platycodon grandiflora group.
[0050] Colon cancer cells were inoculated into 6 cm culture dishes, 5 mL per dish, and then cultured in an incubator. Add 5 mL of complete culture solution to the blank control group, add 5 mL of 10 μM PD drug-containing culture solution to the experimental group, and place them in an incubator to continue culturing for 48 hours. When the cells of the two groups grew to a density of 80%, the cells were collected and the total RNA of the cells was extracted. cDNA was reverse transcribed using a reverse transcription kit.
[0051] The cDNA obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com