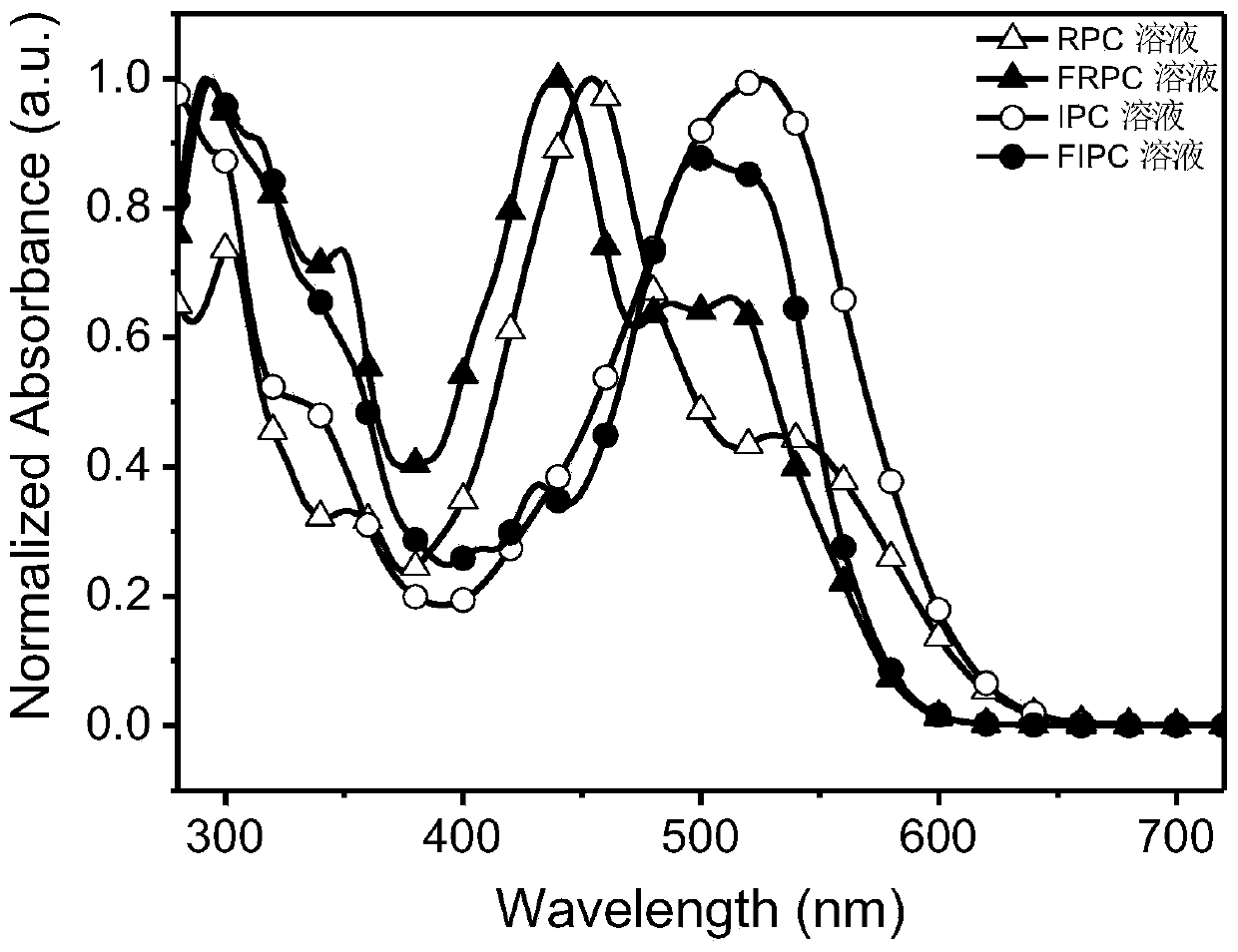
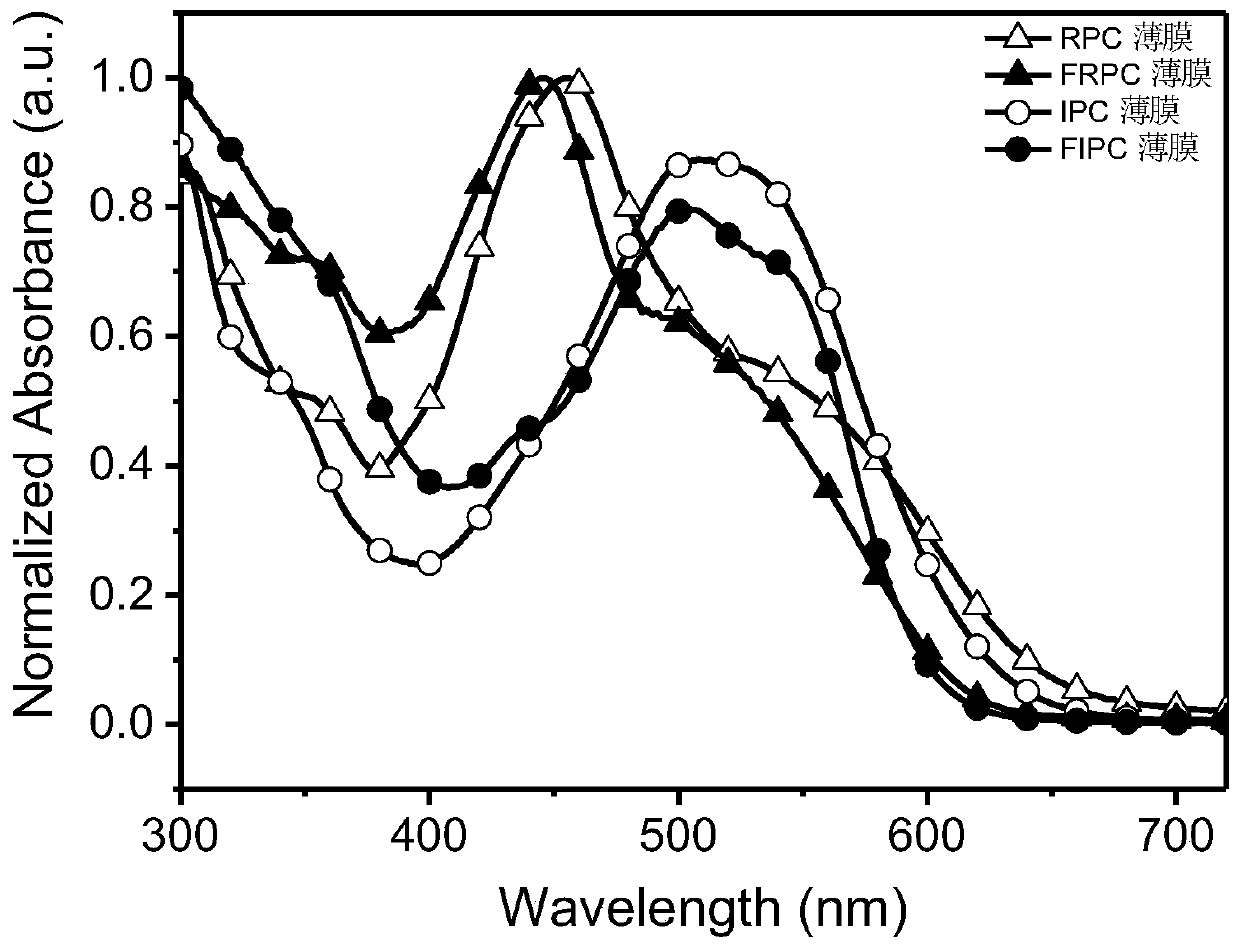
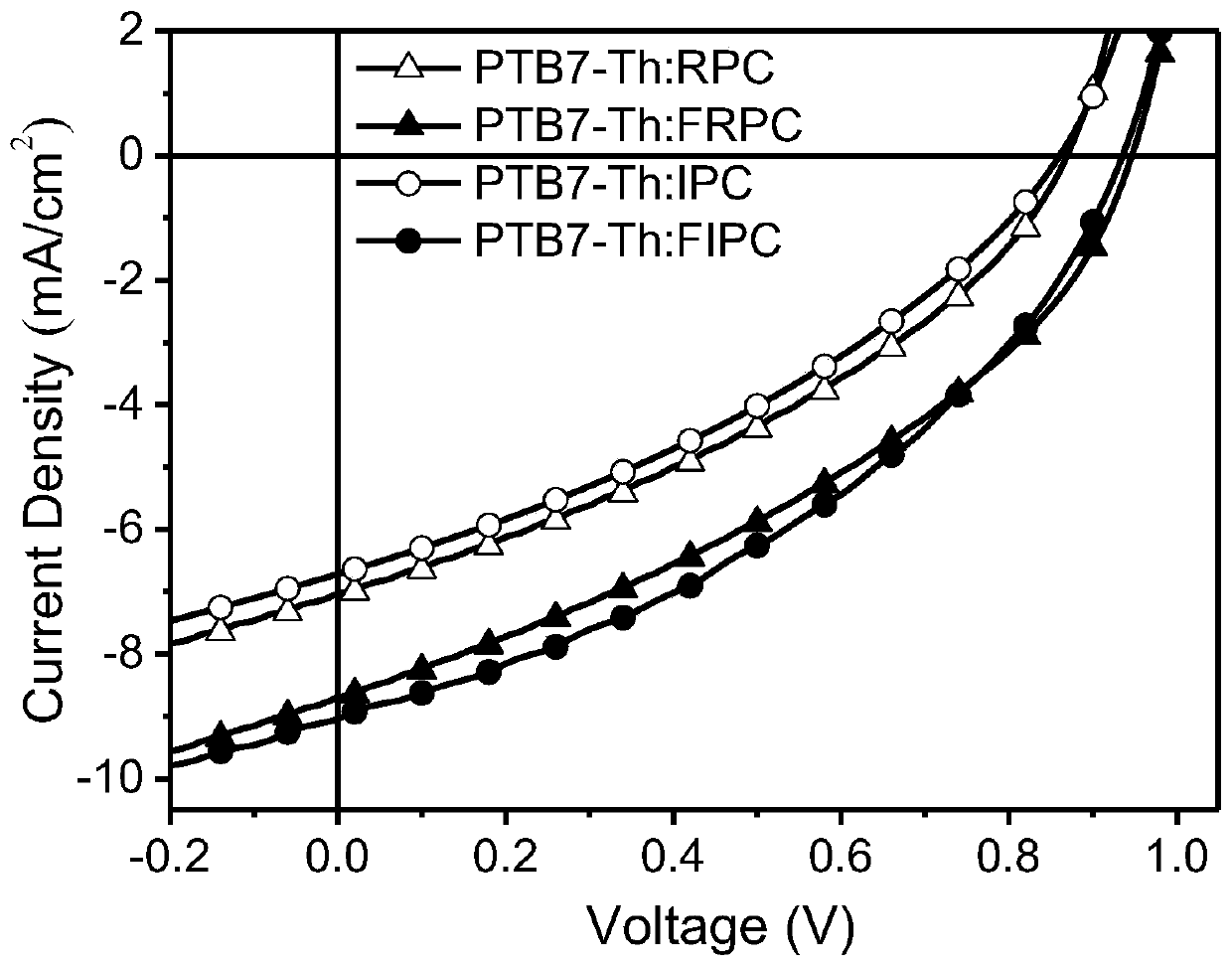
Synthesis of asymmetric non-fullerene receptor and printing preparation of solar battery with asymmetric non-fullerene receptor
A non-fullerene, asymmetric technology, applied in semiconductor/solid-state device manufacturing, circuits, photovoltaic power generation, etc., can solve the problems of cumbersome preparation process, insoluble in organic solvents, high cost, etc., and achieve simple and satisfactory preparation process Energy conversion efficiency and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] An organic small molecule receptor whose chemical structure is RPC provided in the embodiment of the present invention, its synthesis route is as follows:
[0056]
[0057] Compound 1 can be synthesized according to the steps reported in the literature (European journal of organic chemistry, 2006, 2006(17): 4014-4020; Advanced materials, 2014, 26(30): 5137-5142).
[0058] Synthesis of Compound 2: Add Compound 1 (164mg, 0.43mmol), PDI-Br (381mg, 0.46mmol) into a 100mL two-necked flask, and inject 10mL of freshly distilled tetrahydrofuran and 5mL of saturated potassium carbonate into the reaction flask under the protection of argon. The aqueous solution was bubbled for 20 minutes, tetrakis(triphenylphosphine)palladium (25mg, 0.0094mmol) was added, and stirred at 70°C for 12 hours. After being cooled to room temperature, the reaction solution was poured into a one-necked bottle, and the tetrahydrofuran was spinned out under reduced pressure, extracted three times with d...
Embodiment 2
[0063] An organic small molecule receptor with a chemical structure of FRPC provided in the embodiment of the present invention has a synthetic route as follows:
[0064]
[0065] Synthesis of compound 3: In a 100mL two-necked flask, add compound 2 (300mg, 0.30mmol), iodine simple substance (38mg, 0.30mmol), and add 50mL toluene, irradiate the reaction flask with a 400W ultraviolet curing lamp, and raise the temperature to 115 The toluene was refluxed and stirred at ℃, and the end point of the reaction was monitored by TLC thin film chromatography. The reaction was terminated when compound 2 was completely reacted. After returning to room temperature, spin out the toluene under reduced pressure, and directly use column chromatography for purification. The developer is petroleum ether:dichloromethane (v:v=1:3) to obtain a red solid (244mg, 81%) .
[0066] 1 H NMR (400MHz, Chloroform-d) δ10.72 (d, J = 14.96Hz, 1H), 10.06 (s, 2H), 9.27–8.91 (m, 5H), 8.3–8.04 (m, 2H), 7.75 (...
Embodiment 3
[0070] The embodiment of the present invention provides an organic small molecule receptor whose chemical structure is IPC, and its synthesis route is as follows:
[0071]
[0072] Chemical formula is the synthesis of the small molecule acceptor of IPC: in 100mL two-necked flask, add compound 2 (60mg, 0.060mmol), 2-(3-ethyl-4-oxothiazolidine-2-ylidene) malononitrile ( 29mg, 0.18mmol), 10mL chloroform, and 1 drop of piperidine, the temperature was raised to 70°C under reflux and stirred, and the end of the reaction was monitored by TLC thin film chromatography. The reaction was terminated when all reactants 2 had reacted. After returning to room temperature, it was extracted several times with dichloromethane, dried over anhydrous sodium sulfate, filtered, and the crude product was purified by column using petroleum ether:dichloromethane (v:v=1:3) as developing solvent, and then The product was developed and purified on a TLC plate, and recrystallized with dichloromethane / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com