Ganoderma lucidum polysaccharide-based conjugate drug-loaded nano particle with pH/redox dual response and preparation method thereof
A Ganoderma lucidum polysaccharide, drug-loaded nanotechnology, applied in the field of biomedicine and nanomedicine, to achieve good biocompatibility, reduce damage, and improve cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
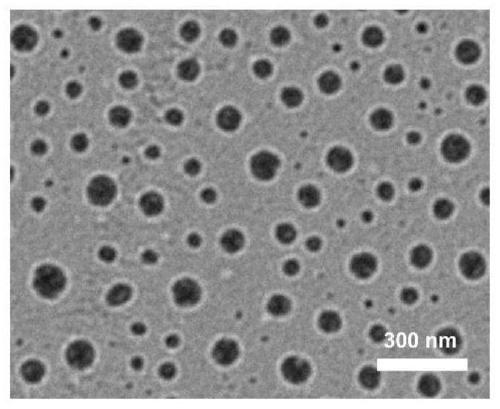
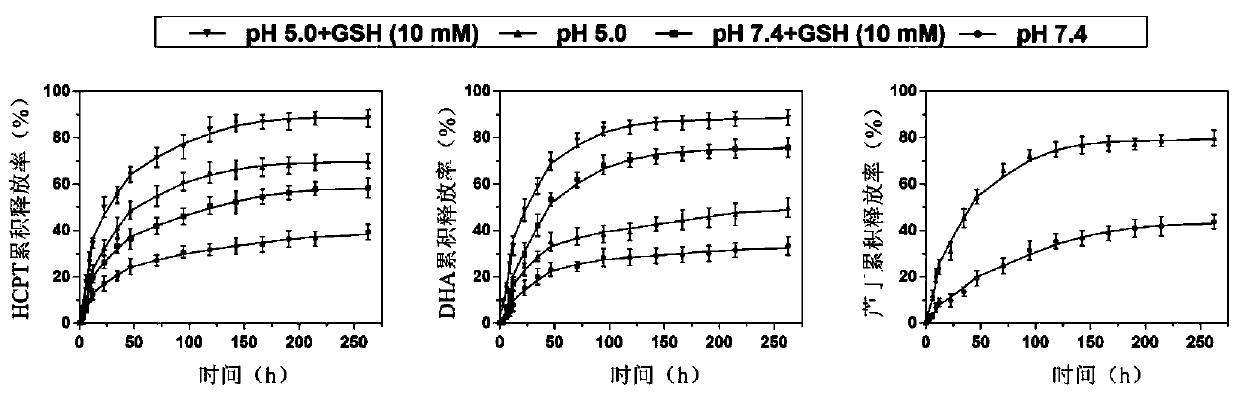
Image
Examples
Embodiment 1
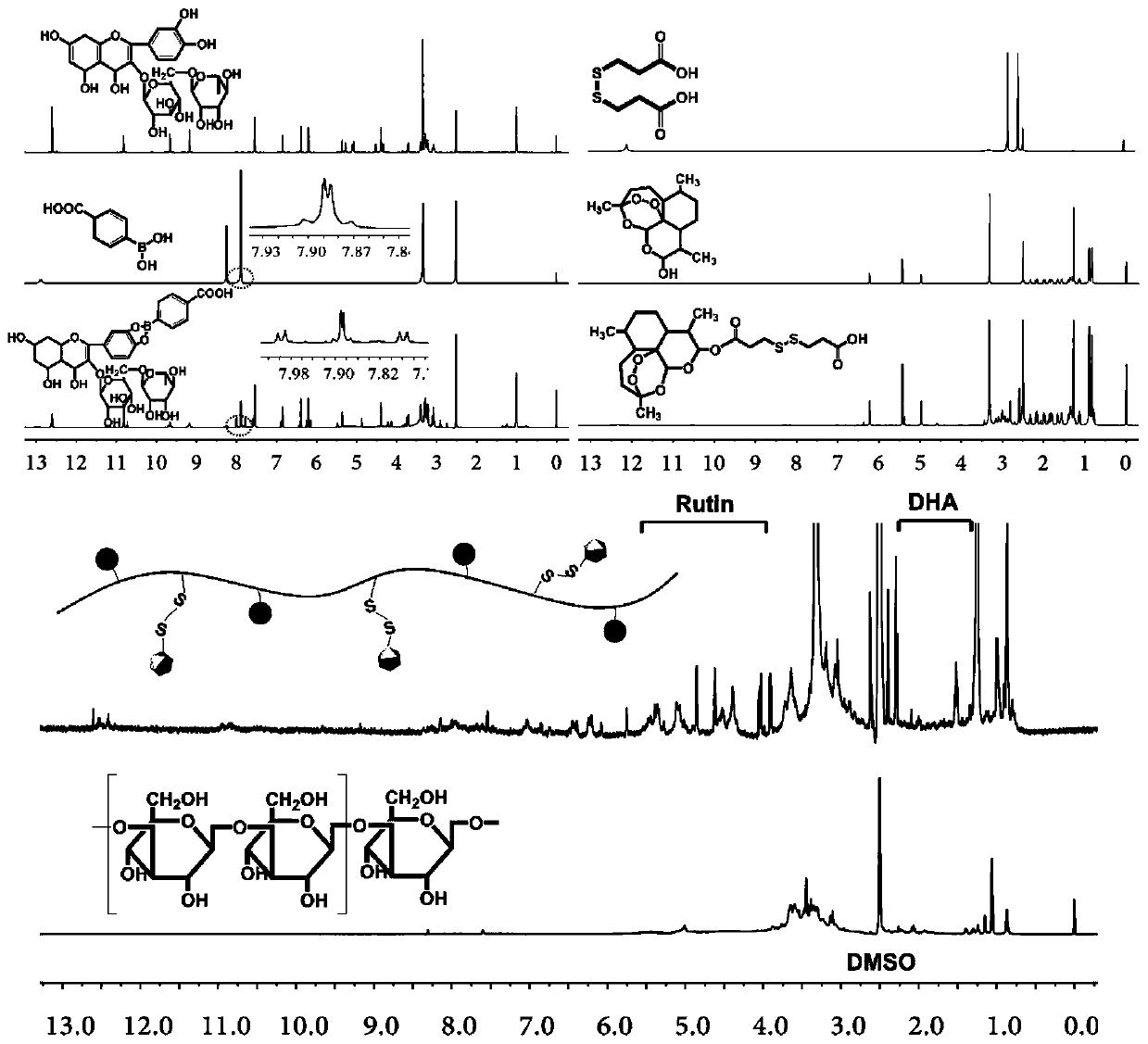
[0030] 1. Weigh 350mg of rutin into a 10mL dry three-necked flask, add 5mL of N,N-dimethylformamide DMF, and continuously inject N into the three-necked flask 2 , add 100mg APBA, and react at room temperature for 24 hours; after the reaction is completed, drop the reaction solution into chloroform to precipitate a precipitate, wash the precipitate three times with chloroform, filter to obtain a solid, and dry the solid in vacuum for 12 hours to obtain a rutin-phenylboronic acid combination thing;
[0031] 2. Weigh 100mg dithiodipropionic acid and dissolve it in 5mL DMSO, add 140mg carbodiimide (EDC) and 90mg N-hydroxysuccinimide (NHS) respectively, activate the carboxyl group at room temperature for 3 hours; add 140mg bis Hydrogen artemisinin and 45mg 4-dimethylaminopyridine (DMAP), at room temperature N 2 React under protection for 24 hours. After the reaction, drop the reaction solution into deionized water, precipitate out, and wash repeatedly with deionized water, redisso...
Embodiment 2
[0035] 1. Weigh 300mg of rutin into a 10mL dry three-necked flask, add 5mL N,N-dimethylformamide DMF, and continuously inject N into the three-necked flask 2 , add 150mg APBA, and react at room temperature for 24 hours; after the reaction is completed, drop the reaction liquid into chloroform to precipitate a precipitate, wash the precipitate three times with chloroform, filter to obtain a solid, and dry the solid in vacuum for 12 hours to obtain rutin-phenylboronic acid combined thing;
[0036] 2. Weigh 150mg dithiodipropionic acid and dissolve it in 5mL DMSO, add 210mg carbodiimide (EDC) and 150mg N-hydroxysuccinimide (NHS) respectively, activate the carboxyl group at room temperature for 3 hours; add 300mg bis Hydrogen artemisinin and 80mg 4-dimethylaminopyridine (DMAP), at room temperature N 2 React under protection for 24 hours. After the reaction, drop the reaction solution into deionized water, precipitate out, and wash repeatedly with deionized water, redissolve the s...
Embodiment 3
[0040] 1. Weigh 450mg of rutin into a 10mL dry three-necked flask, add 3mL of N,N-dimethylformamide DMF, and continuously inject N into the three-necked flask 2 , add 150mg APBA, and react at room temperature for 24 hours; after the reaction is completed, drop the reaction liquid into chloroform to precipitate a precipitate, wash the precipitate three times with chloroform, filter to obtain a solid, and dry the solid in vacuum for 12 hours to obtain rutin-phenylboronic acid combined thing;
[0041] 2. Weigh 120mg dithiodipropionic acid and dissolve it in 5mL DMSO, add 170mg carbodiimide (EDC) and 150mg N-hydroxysuccinimide (NHS) respectively, activate the carboxyl group at room temperature for 3 hours; add 120mg bis Hydrogen artemisinin and 90mg 4-dimethylaminopyridine (DMAP), at room temperature N 2 React under protection for 24 hours. After the reaction, drop the reaction solution into deionized water, precipitate out, and wash repeatedly with deionized water, redissolve th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com