Method for Synthesizing Efficient and Stable All-Inorganic Halogen Perovskite Quantum Dot Scintillators with Equivalent Ligands
A perovskite, quantum dot technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, luminescent materials, etc., can solve problems such as the reduction of quantum efficiency of perovskite quantum dots, and achieve elimination energy Defects, high detection efficiency, and the effect of suppressing non-radiative recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
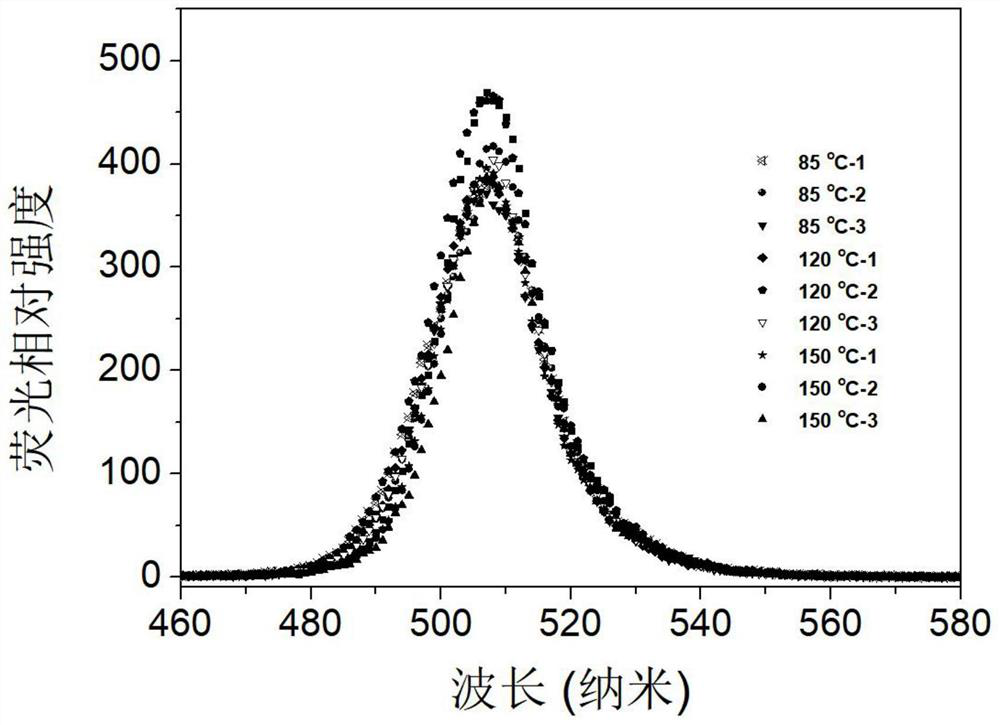
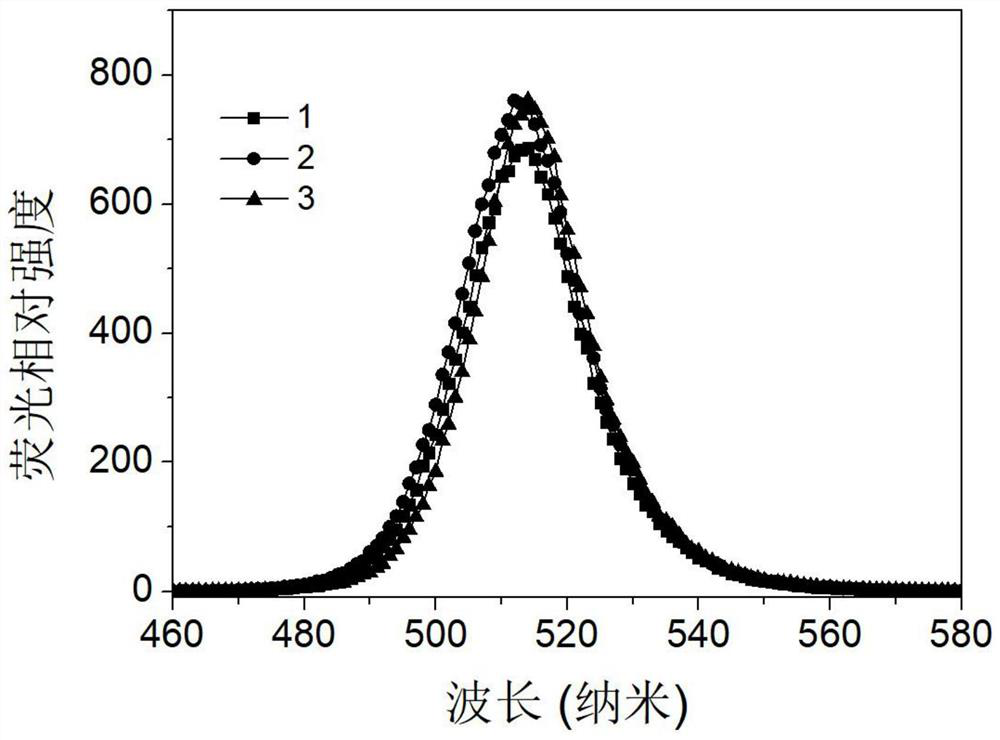
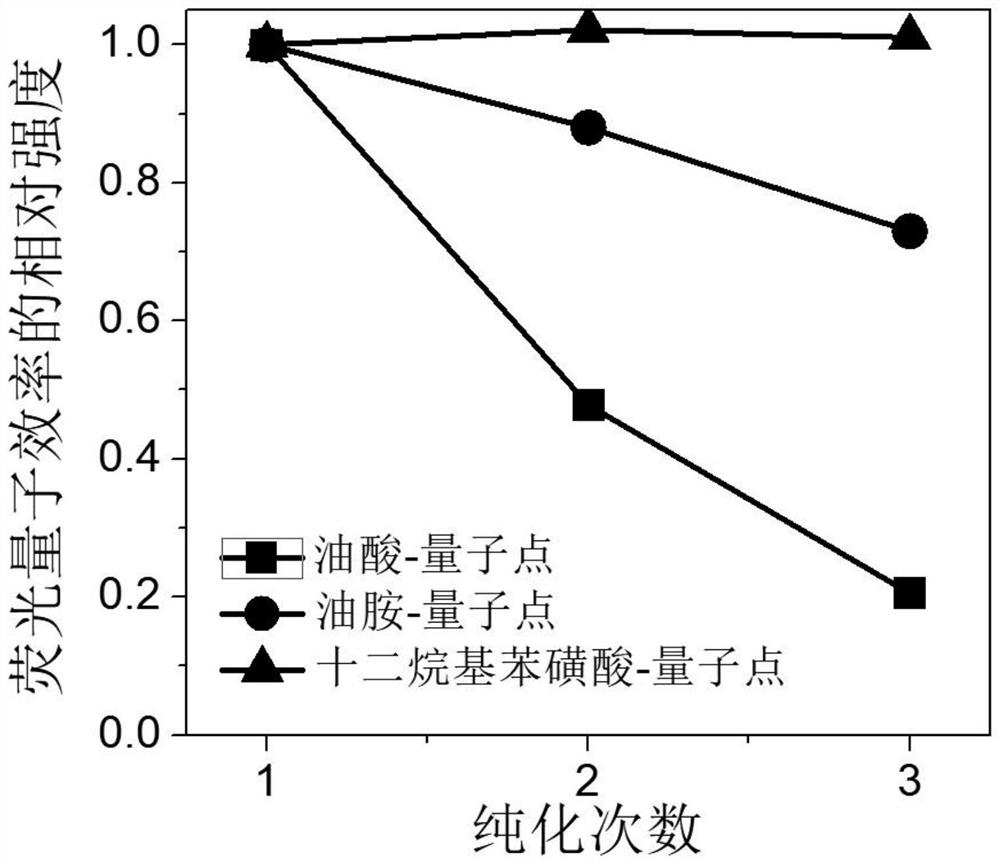
[0031] Preparation of 374 mg / mL of tetraoctylammonium bromide precursor solution of toluene. Weigh 59.3mg of cesium carbonate, 206mg of lead acetate, 10mL octadecyl, 1.2045g of 1,4-dodecylbenzenesulfonic acid solution, added to a 100mL three-mouth flask, the three-mouth flask into the heating sleeve, through the argon degassing, stirring and heating 120 ° C. After the reaction for 30min, cesium carbonate and lead acetate were completely dissolved to form a light brown transparent precursor solution, which was then cooled to 85 °C, and the reaction temperature was stable. The tetraoctyl ammonium bromide precursor solution was quickly injected into the light brown precursor solution, and the reaction was immediately ice bathed for 4 to 10 seconds, stirred vigorously to make it cool down rapidly to obtain an inorganic halogen perovskite quantum dot solution. 30~50mL of ethyl acetate was added to the quantum dot solution, centrifuged at a speed of 7000~9000r / min to collect the preci...
Embodiment 2
[0033] Preparation of a tetraoctyl ammonium bromide precursor solution of 374 mg / mL. Weigh 59.3mg of cesium carbonate, 206mg of lead acetate, 10mL octadecyl and 1.2045g of 1,4-dodecylbenzenesulfonic acid solution added to a 100mL three-mouth flask, the three-mouth flask into the heating sleeve, through argon degassing, stirring and heating 120 ° C. After the reaction for 30min, cesium carbonate and lead acetate were completely dissolved to form a light brown transparent precursor solution, and the reaction temperature was stable. The tetraoctyl ammonium bromide precursor solution was quickly injected into the light brown precursor solution, and the reaction was immediately ice bathed for 4 to 10 seconds, stirred vigorously to make it cool down rapidly to obtain an inorganic halogen perovskite quantum dot solution. 30~50mL of ethyl acetate was added to the quantum dot solution, centrifuged at a speed of 7000~9000r / min to collect the precipitation, dispersed in 3mL toluene solution,...
Embodiment 3
[0035] Preparation of a tetraoctyl ammonium bromide precursor solution of 374 mg / mL. Weigh 59.3mg of cesium carbonate, 206mg of lead acetate, 10mL octadecyl and 1.2045g of 1,4-dodecylbenzenesulfonic acid solution added to a 100mL three-mouth flask, the three-mouth flask into the heating sleeve, through argon degassing, stirring and heating 120 ° C. After the reaction for 30 minutes, cesium carbonate and lead acetate were completely dissolved to form a light brown transparent precursor solution, which was then heated to 150 °C until the reaction temperature was stable. The tetraoctyl ammonium bromide precursor solution was quickly injected into the light brown precursor solution, and the reaction was immediately ice bathed for 4 to 10 seconds, stirred vigorously to make it cool down rapidly to obtain an inorganic halogen perovskite quantum dot solution. 30~50mL of ethyl acetate was added to the quantum dot solution, centrifuged at a speed of 7000~9000r / min to collect the precipitatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com