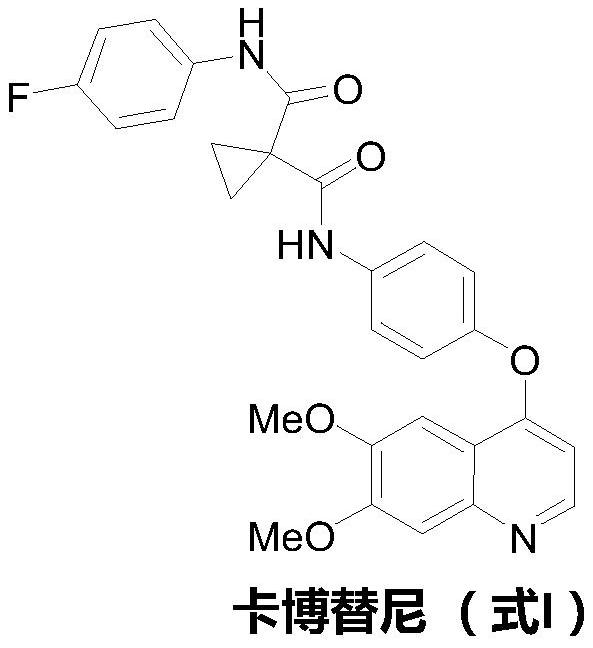
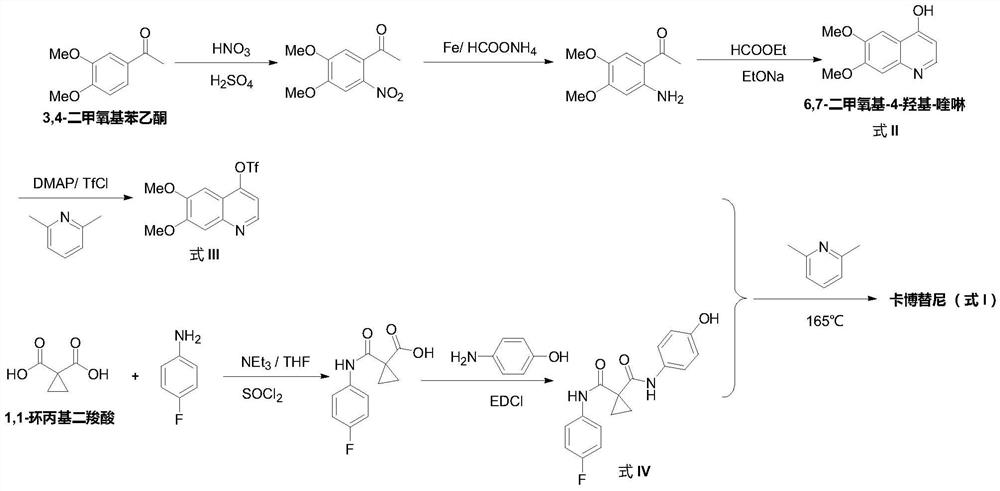
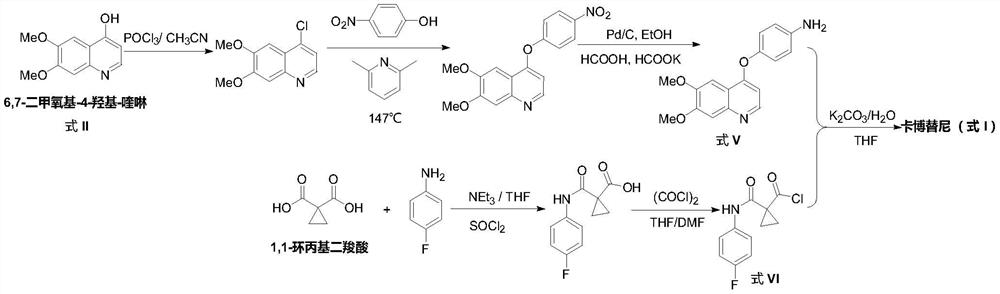
The preparation method of cabozantinib
A cabozantinib, reactive technology, applied in the field of preparation of the drug cabozantinib, can solve the problems of long route, low efficiency, unfavorable waste discharge control, etc., so as to improve reaction efficiency, facilitate industrial production, and reduce emissions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of 6,7-dimethoxy-2-carboxy-quinoline (Formula VII)
[0023] Put 6-nitroveratraldehyde (63.4g, 0.3mol) and THF (380mL) into the 1000mL hydrogenation tank after nitrogen replacement, stir and dissolve, add 5% Pd / C (6.34g, water content 63.5%), nitrogen replacement Afterwards, the pressure of hydrogen gas was introduced to 0.1-0.2 MPa, and the temperature was kept at 25-30° C. for 4 hours. Vent to normal pressure, and filter to remove the catalyst. Transfer the filtrate to a 1000mL four-neck flask, add ethyl pyruvate (38.3g, 0.33mol), raise the temperature to 60°C, add sodium ethoxide solid (30.6g, 0.45mol) in portions, after the addition, keep the reaction at 60-65°C After 4 hours, additional water (200 mL) was added, and the reaction was continued at 60-65° C. for 2 hours. After cooling down, most of the THF was removed by rotary evaporation. The residue was added with water (300mL) and tertiary methyl ether (200mL) to extract the layers. The aqueous layer ...
Embodiment 2
[0025] Preparation of 6,7-dimethoxy-2-carboxy-quinoline (Formula VII)
[0026] 500mL four-necked bottle, put in water (100mL), ethanol (100mL), potassium formate (42.1g, 0.5mol) and formic acid (27.6g, 0.6mol) after nitrogen replacement, add 6-nitroveratraldehyde ( 21.1 g, 0.1 mol), after nitrogen was fully replaced, 5% Pd / C (3.15 g, moisture content 63.5%) was added, and the temperature was slowly raised to 60° C. for 6 h until almost no gas was released. Add methyl pyruvate (11.23g, 0.1mol), raise the temperature to 80-85°C and stir for 30min, then slowly add sodium hydroxide solution (22gNaOH, 100mL water) dropwise, and continue to react at 80-85°C for 2 hours after the dropwise addition. After cooling down, the catalyst was removed by filtration, and the pH value of the filtrate was adjusted to 6-7 with 10% hydrochloric acid, and a large amount of solids were precipitated. Filter, rinse the filter cake with water (50 mL) and suction filter, and air-dry the filter cake at ...
Embodiment 3
[0028] Preparation of 6,7-dimethoxy-2-carboxy-4-chloro-quinoline (formula VIII)
[0029] Add SOCl to 500mL four-necked bottle 2 (150mL), slowly add DMF (3mL) under stirring, then add 6,7-dimethoxy-2-carboxy-quinoline solid (formula VII, 46.6g, 0.2mol), slowly heat up to 75-80°C for reaction After 18 hours, acid gas was released. After cooling down, add toluene (200mL) to dilute the reactant, concentrate under reduced pressure until it is difficult to stir, add toluene (100mL) again and concentrate under reduced pressure until it becomes difficult to stir, add toluene (100mL) to the residue to disperse the solid, cool to 5°C, drop Water (10 mL) was added and the resulting solid-liquid mixture was stirred for 1 hour. Filter, put the filter cake into sodium acetate aqueous solution (sodium acetate 24.6g, water 300mL) for beating and washing for 2 hours, filter, rinse the filter cake fully with water (150mL) and then filter, blow dry the filter cake at 55°C overnight to obtain 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com