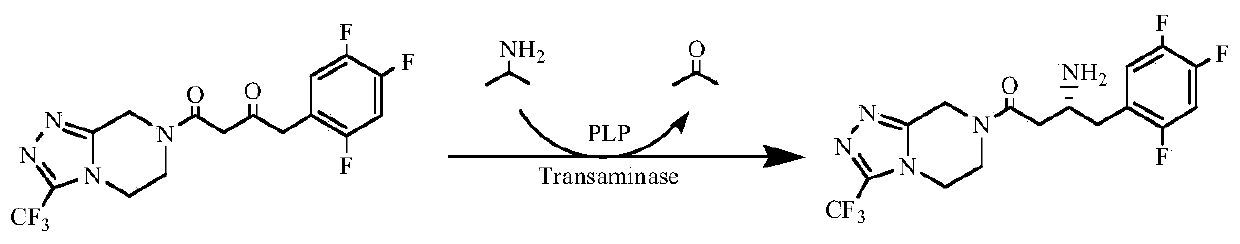
Transaminase-coenzyme co-immobilization engineering bacteria and application
An immobilized cell, transaminase technology, applied in applications, genetic engineering, immobilized on/in organic carriers, etc., can solve the problems of small reaction scale, low substrate concentration, few batch cycles, etc. The effect of high yield and purity, reduction of "three wastes" emissions, and simplified process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Activated carbon pretreatment
[0034] Activated carbon (purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd., analytical grade) was sieved through a 40-mesh sieve. Weigh 100 g of sieved activated carbon and add it to 1000 mL of 1M hydrochloric acid, and stir at 50°C for 1 h. Suction filtration, wash the activated carbon with distilled water and rinse until the filtrate is close to neutral (pH value is 6.9-7.1), dry the filter cake at 60°C, that is, 100g of pretreated activated carbon, store at room temperature for later use.
Embodiment 2
[0035] Embodiment 2: the cultivation of transaminase genetic engineering bacteria cell
[0036] (1) Construction of transaminase genetically engineered bacteria:
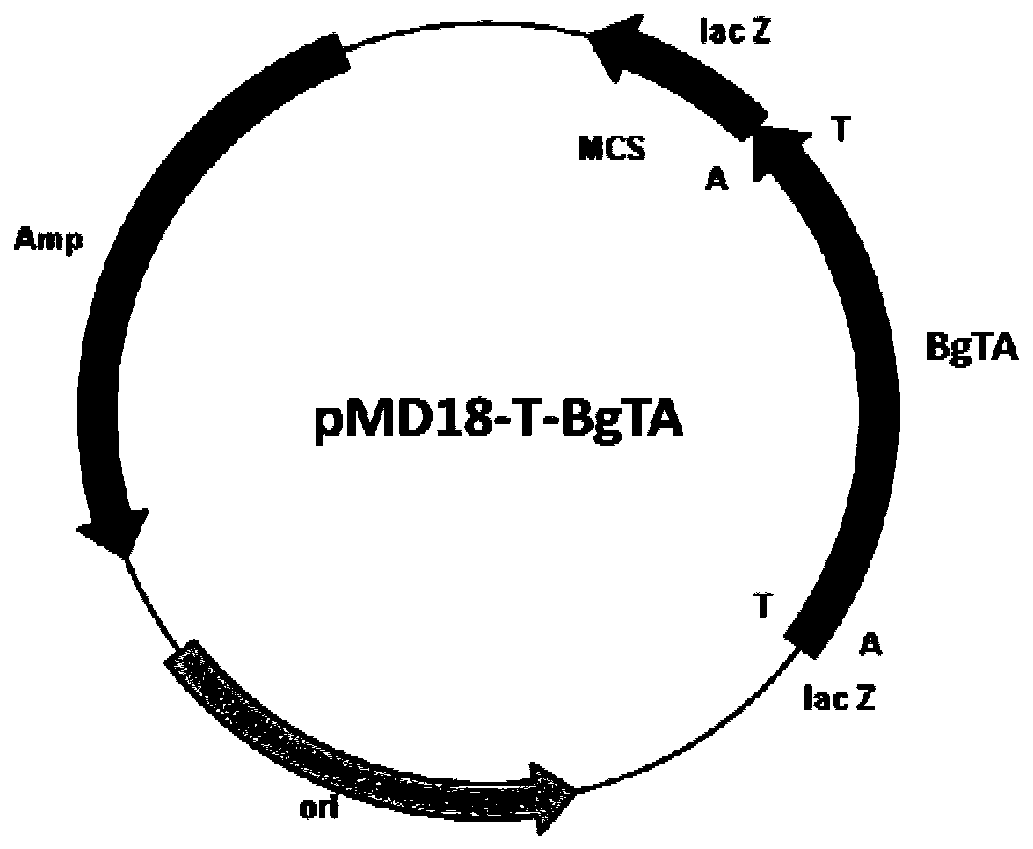
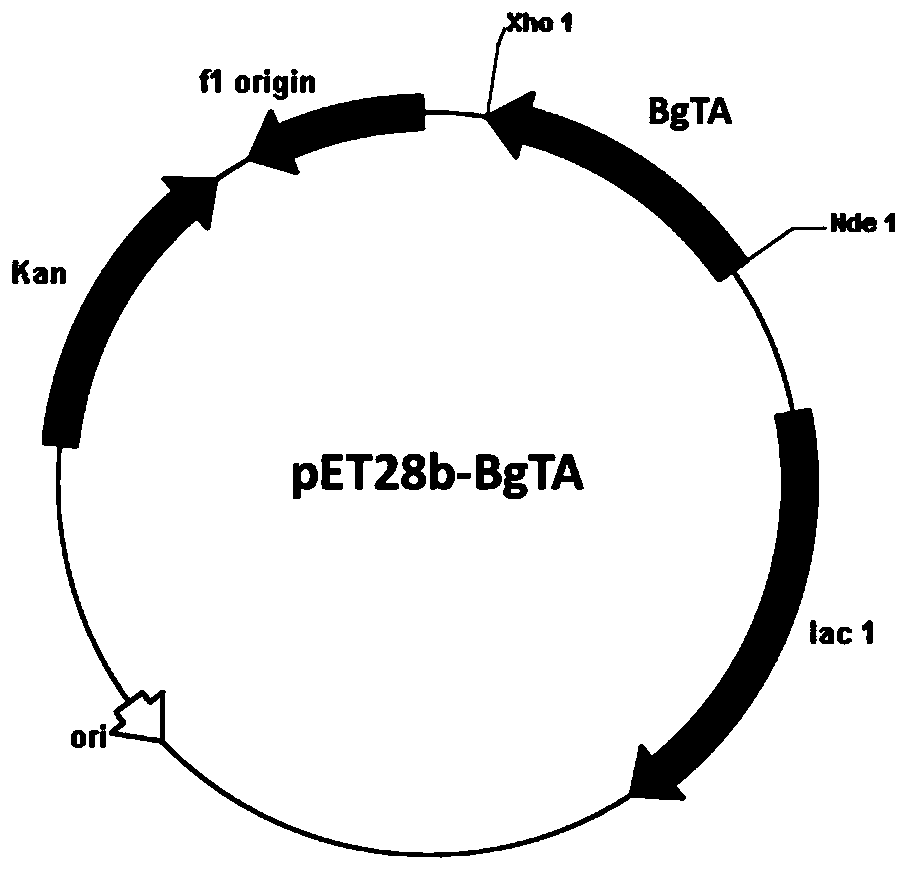
[0037] Primer 1 (CCG CATATG GCTATCATCCAG GTTCAGC), Primer 2 (TTG CTCGAG TCAAGCCGGAACAGAAGAG), and Nde I and Xho I restriction enzyme sites (underlined) were introduced into primer 1 and primer 2, respectively. Under the priming of primer 1 and primer 2, high-fidelity PfuDNA polymerase was used to amplify, and the recombinant plasmid pMD18-T-BgTA( figure 2 ) as a template to obtain the transaminase BgTA gene sequence, after sequencing, use Nde I and Xho I restriction endonuclease (TaKaRa) to treat the amplified fragment, and use T4 DNA ligase (TaKaRa) to treat the amplified fragment with the same restriction The commercial vector pET28b (Invitrogen) treated with endonuclease was connected to construct the expression vector pET28b-BgTA ( image 3 ). The constructed expression vector pET28b-BgTA was transformed...
Embodiment 3
[0040] Example 3: Preparation of transaminase-PLP co-immobilized engineering bacteria cells
[0041] Prepare the glycine buffer solution (molar concentration is 100mM) of pH 9.0 with distilled water, take by weighing 50g of the transaminase genetically engineered bacterium wet thalline prepared by the method of embodiment 2 and join in 1L, pH 9.0, 100mM glycine buffer solution, obtain bacterial suspension 1L . Accurately weigh 0.5g of PLP, add it to 1L of bacterial suspension and mix, at room temperature (20-30°C), 500rpm, stir and adsorb with a water bath stirring paddle for 30min; weigh 10g (dry weight) after the pretreatment of Example 1 Add activated carbon (according to the ratio of 10g / L) to 1L bacterial suspension for mixing, 500rpm water bath stirring paddle stirring and adsorption for 30min; then add 40mL mass concentration 5% polyethyleneimine aqueous solution (according to 4% of the total system volume 1L) , at room temperature, under the condition of 500rpm, stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com