GLP-1 analogue-COL3A1 fusion protein
A GLP-1, fusion protein technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
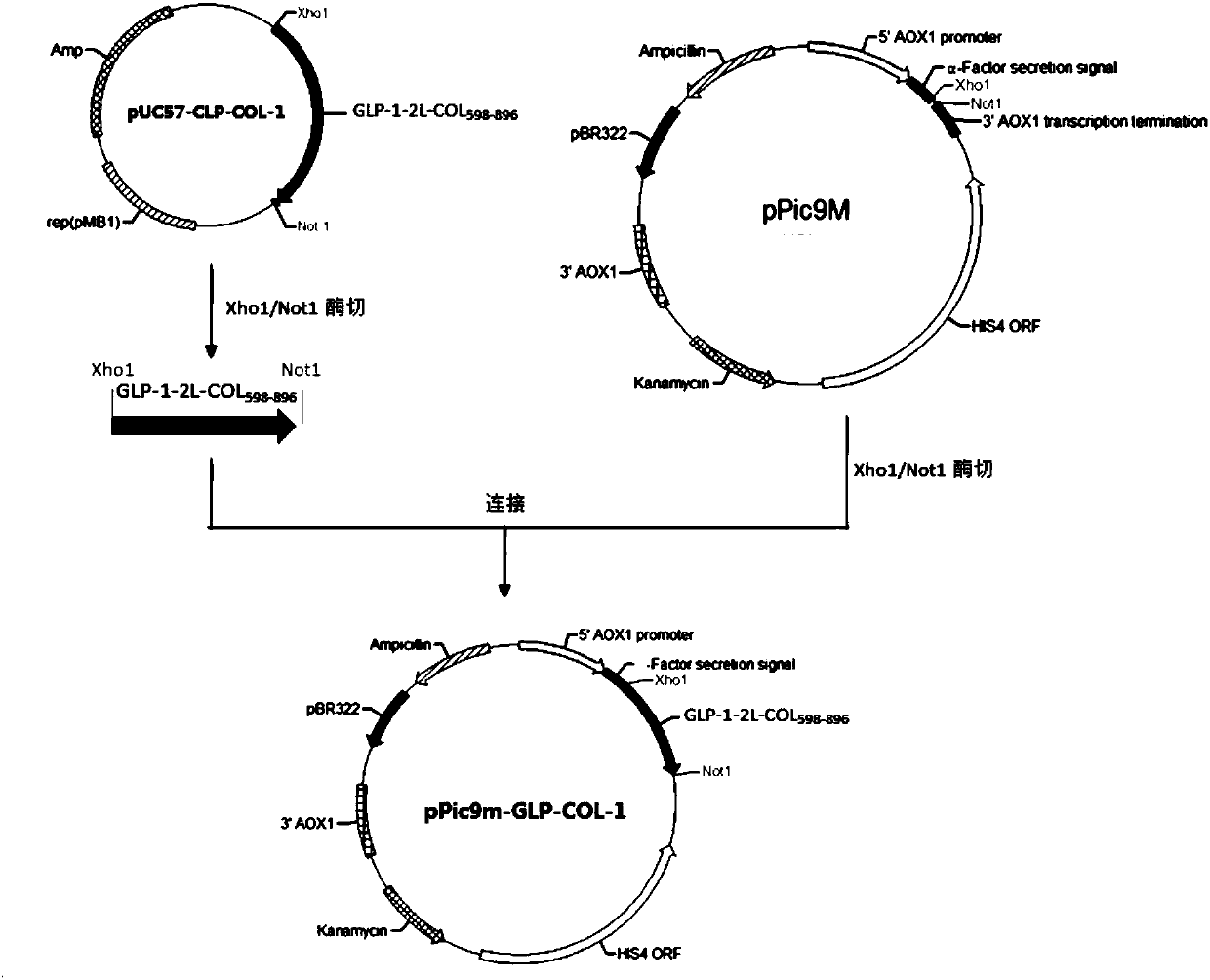
[0134] Example 1: Construction of DNA encoding GLP-1-COL3A1 fusion protein
[0135] Coding fusion protein GLP-1-2L-COL of the present invention 598-896 The gene (SEQ ID NO: 8) was synthesized by Nanjing GenScript Biotechnology Co., Ltd. and cloned into the pUC57 plasmid. The 5' end of the fusion gene contains an XhoI restriction site, and the 3' end contains a TAA stop codon and NotI restriction enzyme site, the pUC57 plasmid was named pUC57-GLP-COL-1.
[0136] Use restriction endonucleases XhoI and NotI (purchased from Fermentas) to carry out double enzyme digestion on pUC57-GLP-COLA-1 according to the instruction manual, and produce the encoded GLP-1-2L-COL with a length of about 1050bp after digestion. 598-896 The gene fragment of the fusion protein was recovered by gel (the gel recovery kit was purchased from Axygen). At the same time, pPic9m was digested with XhoI and NotI, and the digested plasmid band with a length of 9000 bp was gel recovered.
[0137] The fusion pr...
Embodiment 2
[0139] Embodiment 2: Expression of heterologous fusion protein
[0140] The vector inserted with the fusion protein gene in Example 1 was extracted, and transformed into Pichia pastoris GS115 competent cells by electroporation. After screening recombinants by nutrient-limiting medium, G418 resistance was used to screen for high-copy recombinants. Finally, recombinant Pichia pastoris cells containing heterologous fusion protein genes suitable for recombinant expression are obtained.
[0141] Recombinant Pichia pastoris cells were inoculated into a 5L fermenter for small-scale preparation after seed amplification, and the fermentation lasted for 5 days, in which methanol was used to induce the expression of heterologous fusion proteins, and the induction lasted for 36 hours. collected for protein purification.
[0142] Table 1: Composition of Pichia pastoris GS115 seed expansion medium
[0143] formula content Yeast Extract 10.0g / L Peptone 20.0g / L ...
Embodiment 3
[0146] Embodiment 3: Purification of heterologous fusion protein
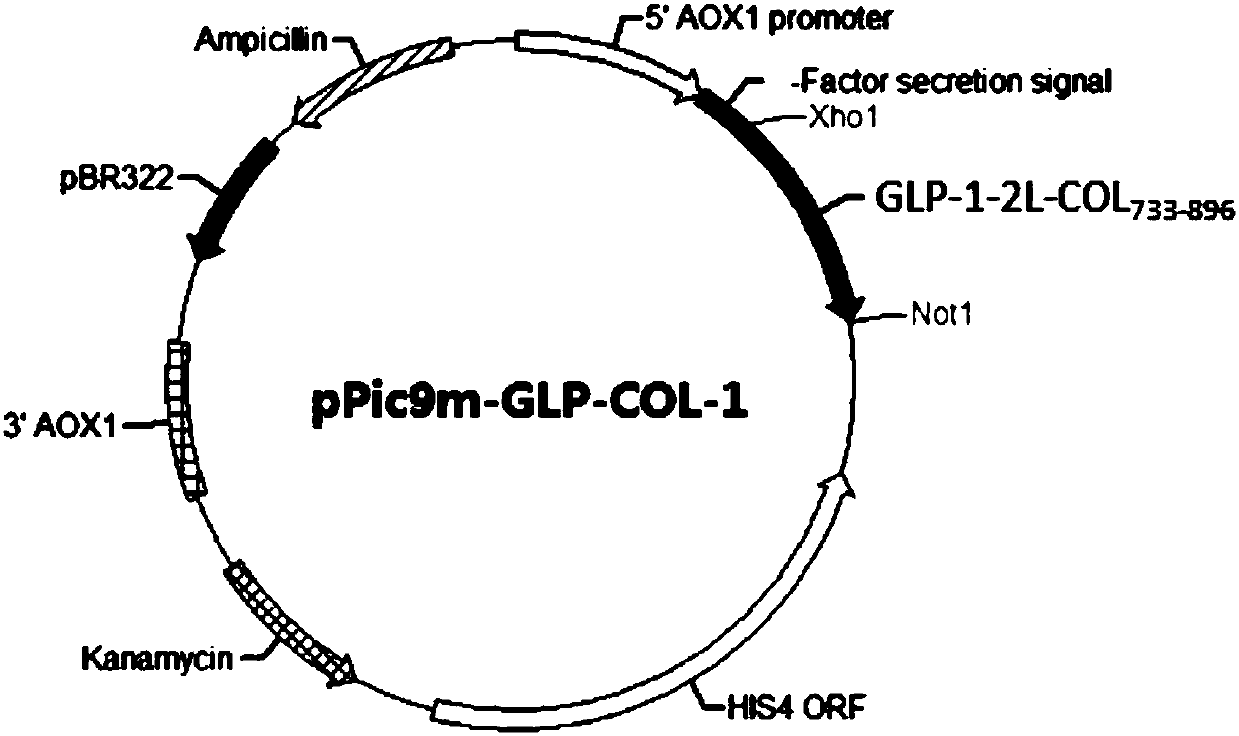
[0147] Two preferred heterologous fusion proteins GLP-1-2L-COL 598-896 , GLP-1-2L-COL 733-896 A similar purification procedure was employed.
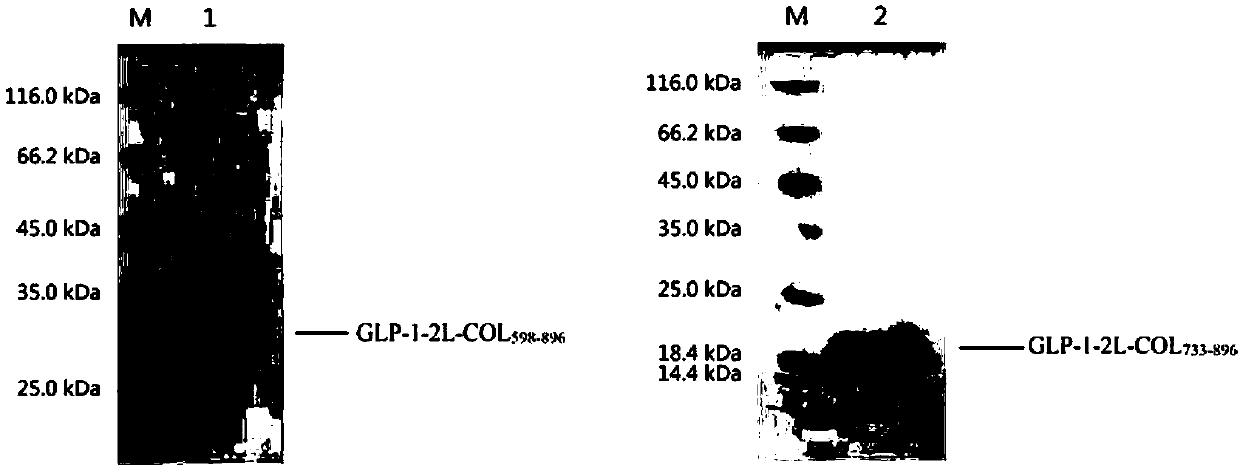
[0148] The 4L fermentation medium was collected using a PALL 0.2μm hollow fiber filtration system to obtain about 4L supernatant. The supernatant was then subjected to sample ultrafiltration with a PALL 50kDa filter membrane ultrafiltration system to remove some impurity proteins. The ultrafiltered liquid was finally purified by Source30Q anion exchange chromatography, and the sample was eluted with a linear gradient of 0-500mM NaCl, and finally the purified heterologous fusion protein was obtained. SDS-PAGE was used to determine the purity and molecular weight of the heterologous fusion protein, the purity was >90%, and the molecular weight was in line with expectations ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com