Method for preparing conjugate
A technology of conjugates and drugs, applied in the field of medicine, can solve problems such as the inability to obtain stable pentafluorophenol esters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
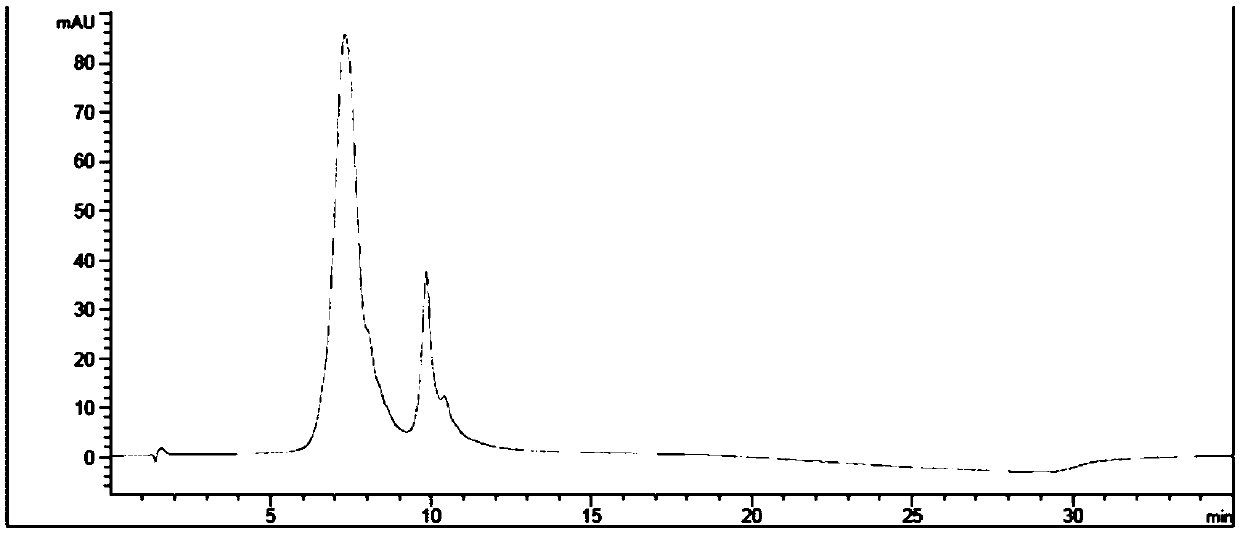
[0250] 1-(4-(((S)-1-(((S)-1-((4-(((S)-2-((2R,3R)-3-((S)-1-( (3R,4S,5S)-4-((S)-2-((S)-2-(Dimethylamino)-3-methylbutanylamino)-N,3-dimethylbutanylamino) -3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropionamido)-3-phenylpropionamido)methyl)phenyl )amino)-1-oxo-5-ureidopent-2-yl)amino)-3-methyl-1-oxobut-2-yl)amino)-4-oxobutyl)-1- Synthesis of Methyl-4-((2,3,5,6-Tetrafluorophenoxy)carbonyl)piperidin-1-ium Iodide (Compound 1)
[0251]
[0252] step one:
[0253] Synthesis of ethyl 1-(4-(tert-butoxy)-4-oxobutyl)piperidine-4-carboxylate (compound 1-2)
[0254] At room temperature, compound 1-1 (1.0g, 6.4mmol) was dissolved in N,N-dimethylformamide (10mL), and tert-butyl 4-bromobutyrate (1.7g, 7.6mmol) was added to the reaction solution ), potassium carbonate (1.77g, 12.8mmol) and potassium iodide (0.53g, 3.2mmol). After addition, stir at room temperature for 5 h, pour the reaction solution into water, extract with ethyl acetate (20 mL×3), wash with saturate...
Embodiment 2
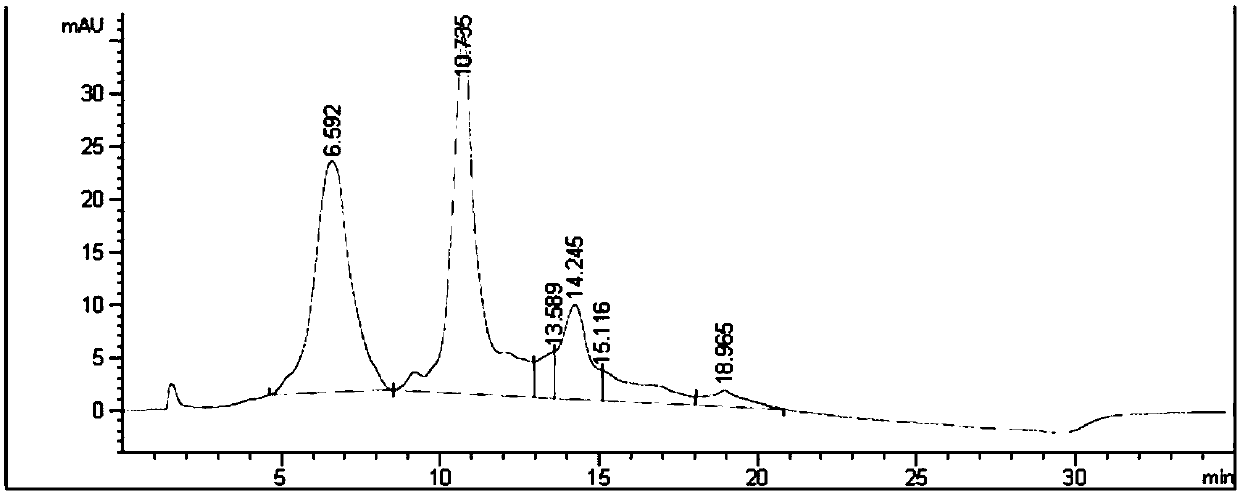
[0271] 1-(4-(((S)-1-(((S)-1-((4-(((S)-2-((2R,3R)-3-((S)-1-( (3R,4S,5S)-4-((S)-2-((S)-2-(Dimethylamino)-3-methylbutanylamino)-N,3-dimethylbutanylamino) -3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropionamido)-3-phenylpropionamido)methyl)phenyl )amino)-1-oxo-5-ureidopent-2-yl)amino)-3-methyl-1-oxobut-2-yl)amino)-4-oxobutyl)-4- Synthesis of ((2,4,6-trifluorophenoxy)carbonyl)piperidine-1-oxide (Compound 2)
[0272]
[0273] step one:
[0274] Synthesis of 1-(4-(tert-butoxy)-4-oxobutyl)-4-(ethoxycarbonyl)piperidine-1-oxide (compound 2-2)
[0275] Compound 1-2 (700 mg, 2.34 mmol) was dissolved in dichloromethane (10 mL) at room temperature, m-chloroperoxybenzoic acid (808 mg, 4.68 mmol) was added under nitrogen protection, and stirred overnight at room temperature. Use high-performance liquid chromatography-mass spectrometry to monitor the complete reaction of the raw materials, the reaction solution is quenched with saturated aqueous sodium bicarbonate, ...
Embodiment 3
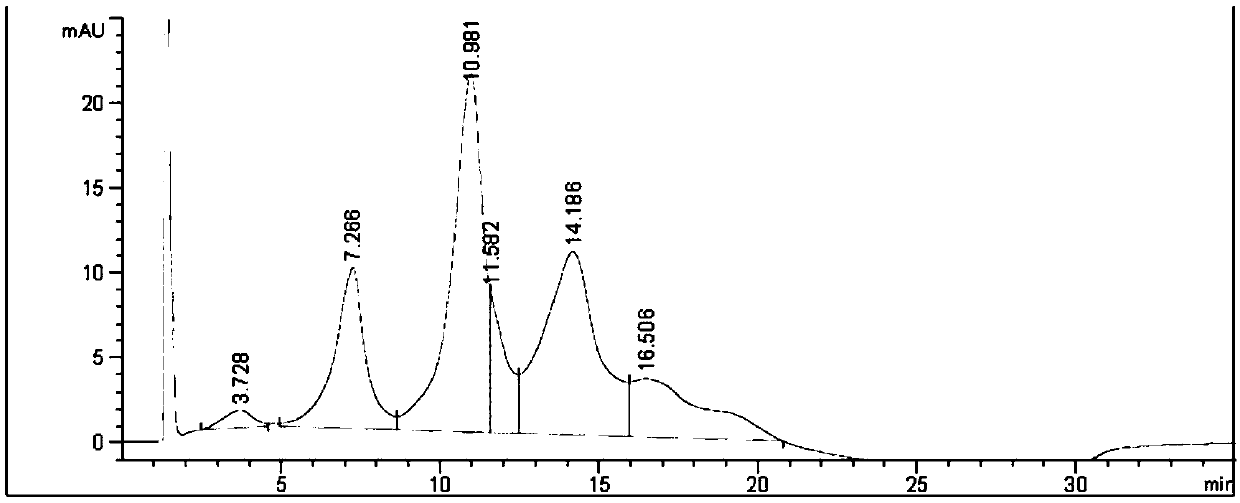
[0289] 1-(4-(((S)-1-(((S)-1-((4-(((S)-2-((2R,3R)-3-((S)-1-( (3R,4S,5S)-4-((S)-2-((S)-2-(Dimethylamino)-3-methylbutanylamino)-N,3-dimethylbutanylamino) -3-methoxy-5-methylheptanoyl)pyrrolidin-2-yl)-3-methoxy-2-methylpropionamido)-3-phenylpropionamido)methyl)phenyl )amino)-1-oxo-5-ureidopent-2-yl)amino)-3-methyl-1-oxobut-2-yl)amino)-4-oxobutyl)-1- Synthesis of Methyl-4-((2,4,6-trifluorophenoxy)carbonyl)piperidin-1-ium Iodide (Compound 3)
[0290]
[0291] Using the similar operation described in Step 6 of Example 1, substituting 2,4,6-trifluorophenol for 2,3,5,6-tetrafluorophenol, 21 mg of the title compound was obtained. ESI-MS(m / z):1448.2[M] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com