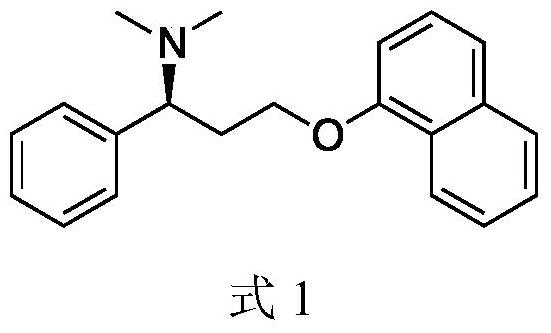
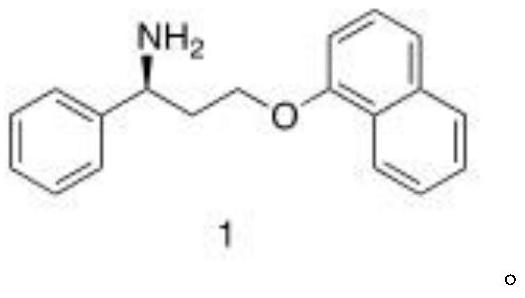
A kind of biosynthesis method of dapoxetine intermediate and intermediate thereof
A technology for biosynthesis and dapoxetine, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of being unable to be suitable for large-scale industrial production, low product yield, and high cost, and achieve synthesis The effect of novel route, good product purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound (4)
[0034] Under room temperature conditions, add compound (2) 1.69kg (10mol) and compound (3) 1.59kg (11mol) in 7L dichloromethane and 5L water in 20L reactor, add catalyst tetrabutylammonium bromide 16g (0.05 mol) and benzyltriethylammonium chloride 12g (0.05mol), after the TLC monitoring reaction was completed (42 hours), the reaction liquid was separated, and the organic phase was washed with 5% aqueous sodium bicarbonate solution 5L, and the organic phase was Concentration under pressure (0.02 mmHg) gave 2.68 kg (9.7 mol) of compound (4), with a molar yield of 97%, and a purity of 98.6% by HPLC.
[0035] 1 H NMR (400MHz, DMSO-d 6 )δ8.14–7.94(m,2H),7.71–7.24(m,9H),7.01–6.59(m,1H),4.13(t,J=7.8Hz,2H),2.97(t,J=7.8Hz ,2H).
[0036] ESI+[M+H] + =277.
[0037] Preparation of compound (1)
[0038] At 25°C, add the reaction solvent to the reaction kettle, then add the ammonia source, adjust the pH to 8.0 with hydrochloric acid, then add PLP ...
Embodiment 2
[0047] According to the synthetic method of embodiment 1, difference is: in the preparation of compound (4), the mol ratio of compound (2) and compound (3) is 1:1.2, and reaction temperature is 50 ℃, and reaction solvent is toluene and water ( Volume ratio 1:1), the molar ratio of compound (2) and catalyst tetrabutyl ammonium chloride is 200:20; Molar yield is 95.2%. Purity by HPLC: 97.2%.
[0048] In the preparation of compound (1), the reaction solvent is water / ethanol, the ammonia source is butylamine, and the concentration is 1.25M; the mass concentration of compound (4) is 150g / L; the compound (4) and biological enzyme (S152E, L161V) The mass concentration ratio is 75:1; the mass yield is 95.5%, and the HPLC detection purity is 97.8%.
Embodiment 3
[0050] According to the synthetic method of Example 1, the difference is that in the preparation of compound (4), the molar ratio of compound (2) and compound (3) is 1:1, the reaction temperature is 0 ° C, the reaction solvent is tetrahydrofuran, compound ( 2) The molar ratio to the catalyst benzyltriethylammonium chloride is 200:1; the molar yield is 92.1%. Purity by HPLC: 95.6%.
[0051] In the preparation of compound (1), the reaction solvent is water / methanol, the ammonia source is propylamine, and the concentration is 0.25M; the mass concentration of compound (4) is 2g / L; the mass concentration ratio of compound (4) and biological enzyme (ABJ05767) The ratio is 1:1; the mass yield is 90.5%, and the HPLC detection purity is 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com