A kind of preparation method of pharmaceutical intermediate
An intermediate and pharmaceutical technology, applied in the field of chemical and pharmaceutical intermediates, to achieve the effects of cheap and easy-to-obtain raw materials, novel synthetic routes, and simple operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
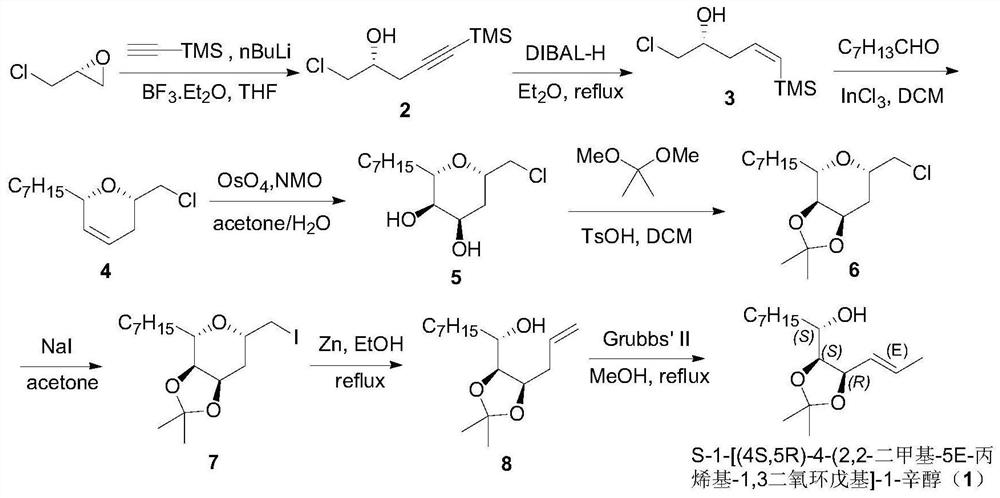
[0026] The present invention starts from the raw material (R)-epichlorohydrin, and provides a pharmaceutical intermediate (S)-1-[(4S,5R)-4-(2,2 - a preparation method of dimethyl-5E-propenyl-1,3-dioxol (1,3-dioxol)]-1-octanol (1), which comprises 8 steps of reaction in total.
[0027] (a) Preparation of (R)-1-chloro-5-trimethylsilyl-4-pentyn-2-alcohol:
[0028] Under anhydrous and oxygen-free conditions, dissolve trimethylsilylacetylene (5.82g, 0.06mol) in anhydrous tetrahydrofuran (250mL), add butyllithium (2.5M, 24mL, 0.06mol) dropwise at minus 78 degrees, and stir After 1 hour, add boron trifluoride diethyl ether (0.06mol), and after 10 minutes, add the raw material R-epichlorohydrin (4.85g, 0.05mol), keep the temperature for 2 hours, then raise the temperature to minus 30 degrees, and keep the reaction for 19 After 1 hour, TLC detected that the reaction of the raw materials was complete, adding saturated ammonium chloride to quench the reaction, distilling off the solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com