A method for preparing 2-chloro-3,3,3-trifluoropropene
A technology of trifluoropropene and trichloropropene, which is applied in the field of preparation of 2-chloro-3,3,3-trifluoropropene, which can solve the problems of easy carbon formation, reactor clogging, and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 oxychlorination catalyst
[0037] Weigh out 125 g of CuCl 2 2H 2 O, 63 grams of MgCl 2 Dissolve in 1000ml of deionized water to make solution A; weigh 40 grams of aluminum hydroxide powder, add it to the sodium hydroxide solution at 50°C with stirring, and stir for 15 minutes to make solution B; add solution A to the solution while stirring In B, stir for 20 minutes after adding, then precipitate, wash with deionized water until the conductivity is greater than 400Ω·cm -1 , and then filtered and dried; the washed catalyst particles are now dried at 150°C for 2 hours, and then roasted at 450°C for 2 hours, and finally the dried catalyst particles are made into a cylindrical shape, ready for use.
Embodiment 2
[0038] Example 2 Preparation of HCFC-1233xf
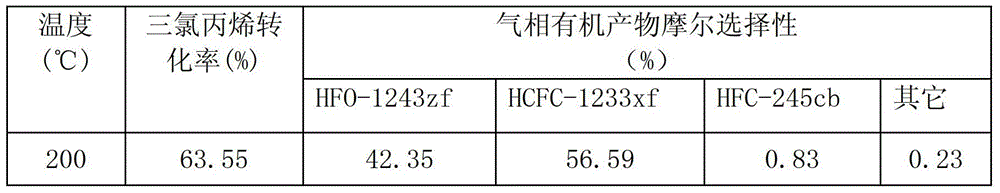
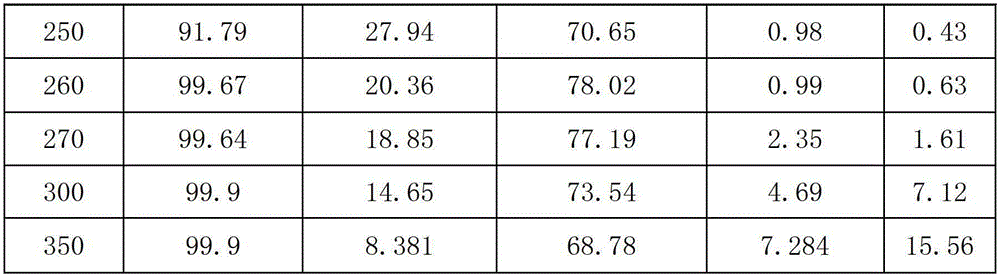
[0039] Raw material molar ratio is CCl 2 =CHCH 2 Cl:HCl:O 2 :HF=1:1.2:2.4:10, reaction temperature 200-350℃, reaction pressure 0.3MPa, space velocity 400h -1 , The oxychlorination catalyst is as shown in example 1, and the loading amount is 200ml, and the loading amount of the fluorination catalyst is 200ml.
[0040] Table 1
[0041]
[0042]
Embodiment 3
[0043] Example 3 Preparation of HCFC-1233xf
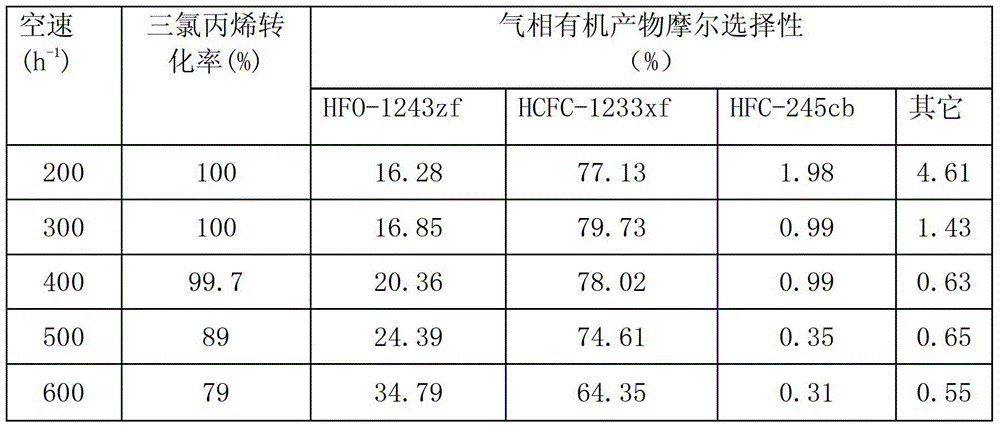
[0044] Raw material molar ratio is CH 2 =CHCCl 3 :HCl:O 2 :HF=1:1.2:2.4:10, the reaction temperature is 260°C, the reaction pressure is 0.3MPa, the space velocity of the raw material relative to the oxychlorination catalyst and the fluorination catalyst is 200-600h -1 , The oxychlorination catalyst is as shown in example 1, and the loading amount is 200ml, and the loading amount of the fluorination catalyst is 200ml.
[0045] Table 2
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com