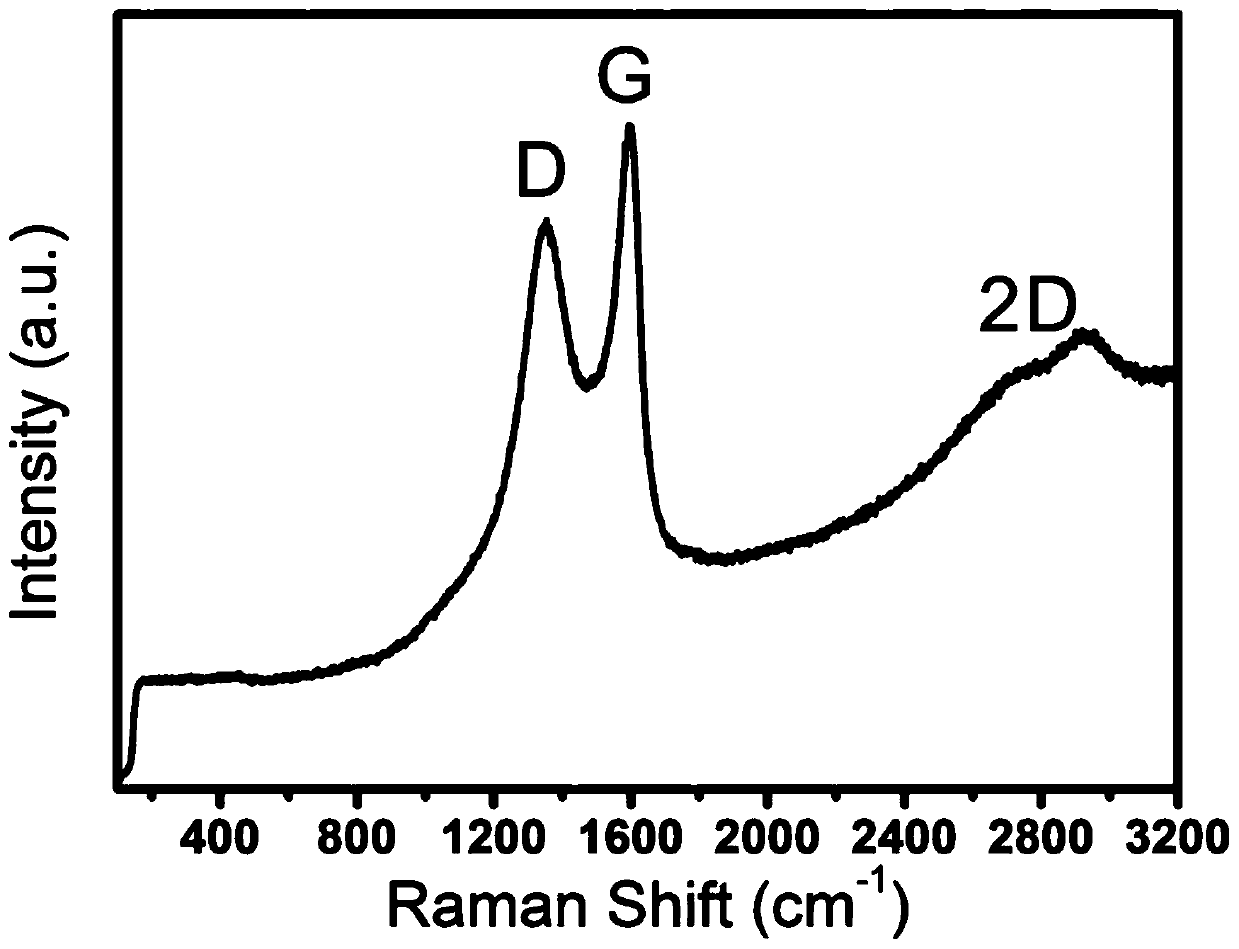
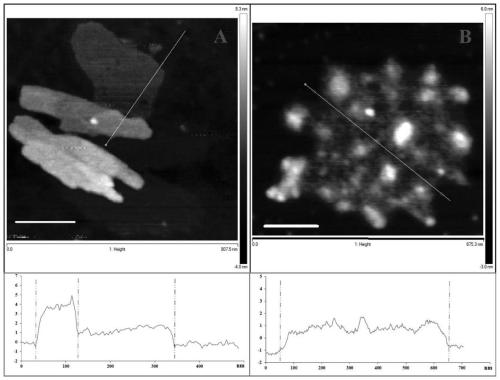
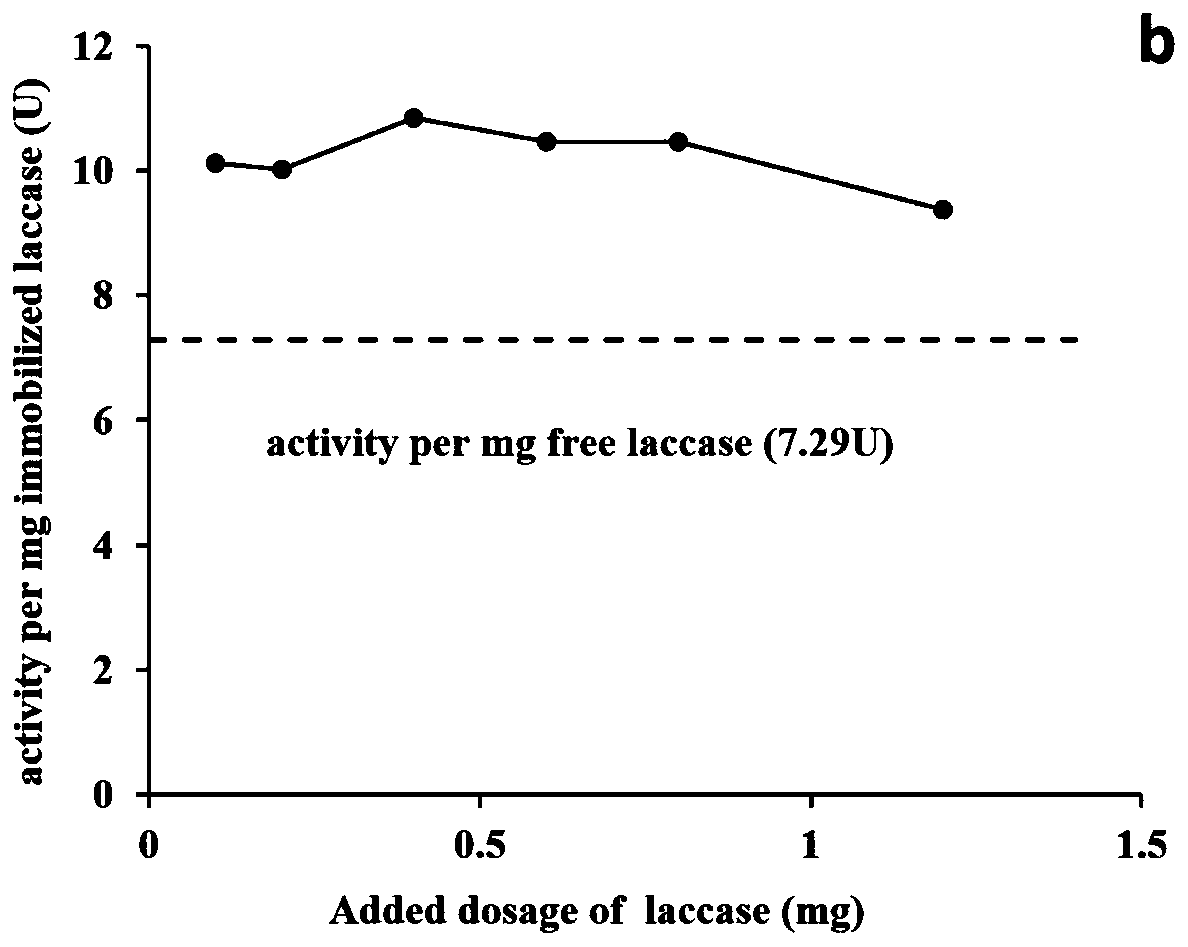
Preparation method of graphene immobilized laccase
A technology of graphene and laccase, which is applied to biochemical equipment and methods, and enzymes immobilized on or in inorganic carriers, can solve the problems of expensive synthesis, complicated processing technology, and poor separation effect, and achieve enzyme retention Live, improve stability, avoid chemical damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Carrier preparation, its steps are:
[0027] (1) Accurately weigh 5g of dodecylamine powder, dissolve it in 20ml of absolute ethanol, weigh 1.590g of ferrous chloride tetrahydrate and 0.900g of ammonium dihydrogen phosphate and dissolve it in 80ml of ultrapure water (dihydrogen phosphate The molar ratio of ammonium to ferrous chloride tetrahydrate is 1:1.02, and the molar ratio of ammonium dihydrogen phosphate to dodecylamine is 1:3.45). In a water bath at 45°C, mix the anhydrous ethanol solution of dodecylamine with the mixed solution of ferrous chloride and ammonium dihydrogen phosphate, collect the solid product by centrifugation, and dry it in vacuum at 40°C for 36 hours. The dried solid product was ground into a powder and placed in a crucible. After drying, put the crucible into a tube furnace, and calcine at 600°C for 24 hours under a low-flow argon protective atmosphere;
[0028] (2) After the calcination process is over, after the product is cooled to room te...
Embodiment 2
[0033] Carrier preparation, its steps are:
[0034] (1) Accurately weigh 5g of dodecylamine powder, dissolve it in 20ml of absolute ethanol, weigh 1.590g of ferrous chloride tetrahydrate and 0.900g of ammonium dihydrogen phosphate and dissolve it in 80ml of ultrapure water (dihydrogen phosphate The molar ratio of ammonium to ferrous chloride tetrahydrate is 1:1.02, and the molar ratio of ammonium dihydrogen phosphate to dodecylamine is 1:3.45). In a water bath at 45°C, mix the anhydrous ethanol solution of dodecylamine with the mixed solution of ferrous chloride and ammonium dihydrogen phosphate, collect the solid product by centrifugation, and dry it in vacuum at 40°C for 36 hours. The dried solid product was ground into a powder and placed in a crucible. After drying, put the crucible into a tube furnace, and calcine at 600°C for 24 hours under a low-flow argon protective atmosphere;
[0035](2) After the calcination process is over, after the product is cooled to room tem...
Embodiment 3
[0040] Carrier preparation, its steps are:
[0041] (1) Accurately weigh 5g of dodecylamine powder, dissolve it in 20ml of absolute ethanol, weigh 1.590g of ferrous chloride tetrahydrate and 0.900g of ammonium dihydrogen phosphate and dissolve it in 80ml of ultrapure water (dihydrogen phosphate The molar ratio of ammonium to ferrous chloride tetrahydrate is 1:1.02, and the molar ratio of ammonium dihydrogen phosphate to dodecylamine is 1:3.45). In a water bath at 45°C, mix the anhydrous ethanol solution of dodecylamine with the mixed solution of ferrous chloride and ammonium dihydrogen phosphate, collect the solid product by centrifugation, and dry it in vacuum at 40°C for 36 hours. The dried solid product was ground into a powder and placed in a crucible. After drying, put the crucible into a tube furnace, and calcine at 600°C for 24 hours under a low-flow argon protective atmosphere;
[0042] (2) After the calcination process is over, after the product is cooled to room te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com