GPR1 antagonistic polypeptide and derivative and application thereof
A derivative, antagonistic technology used in biotechnology and biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
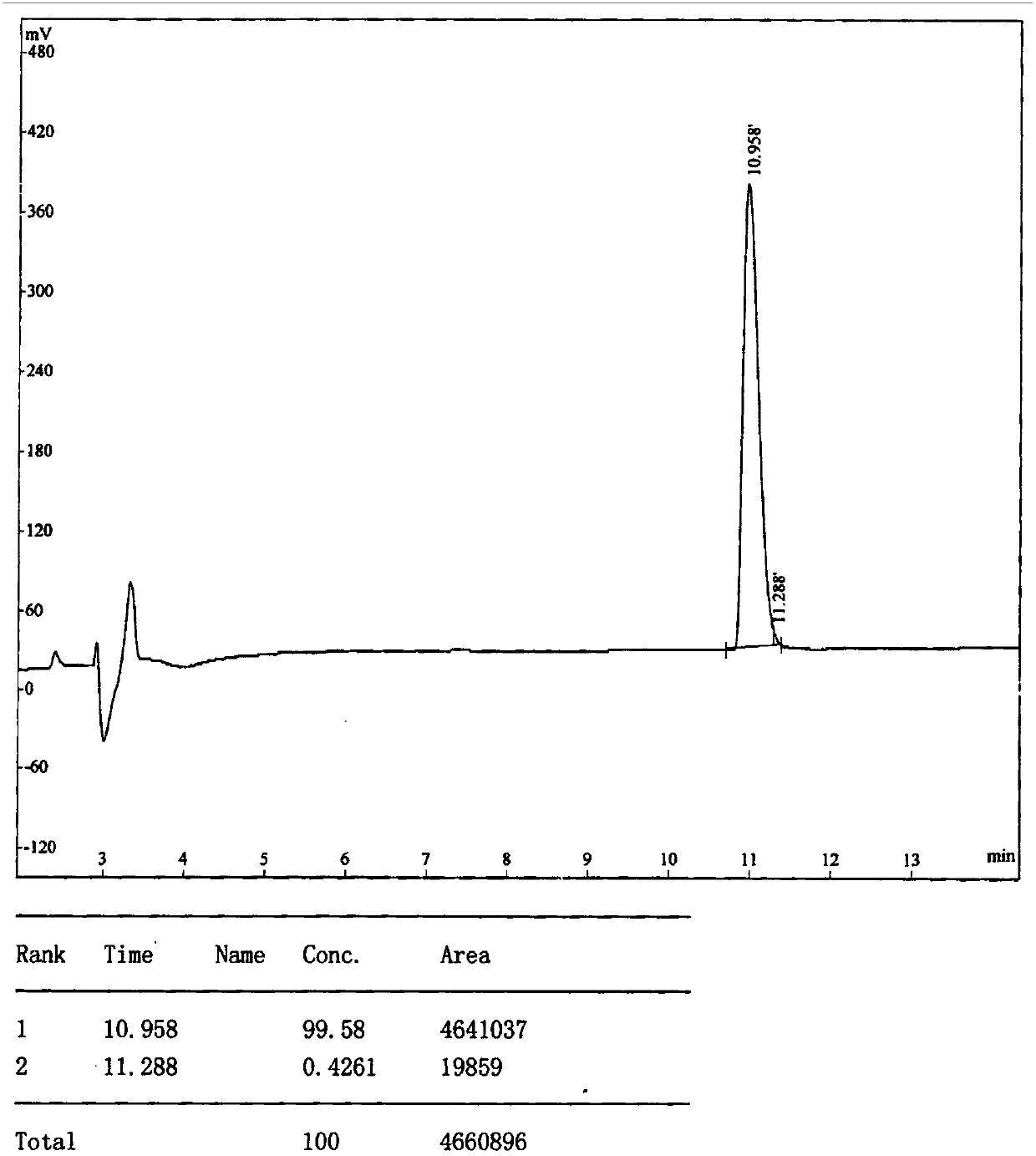
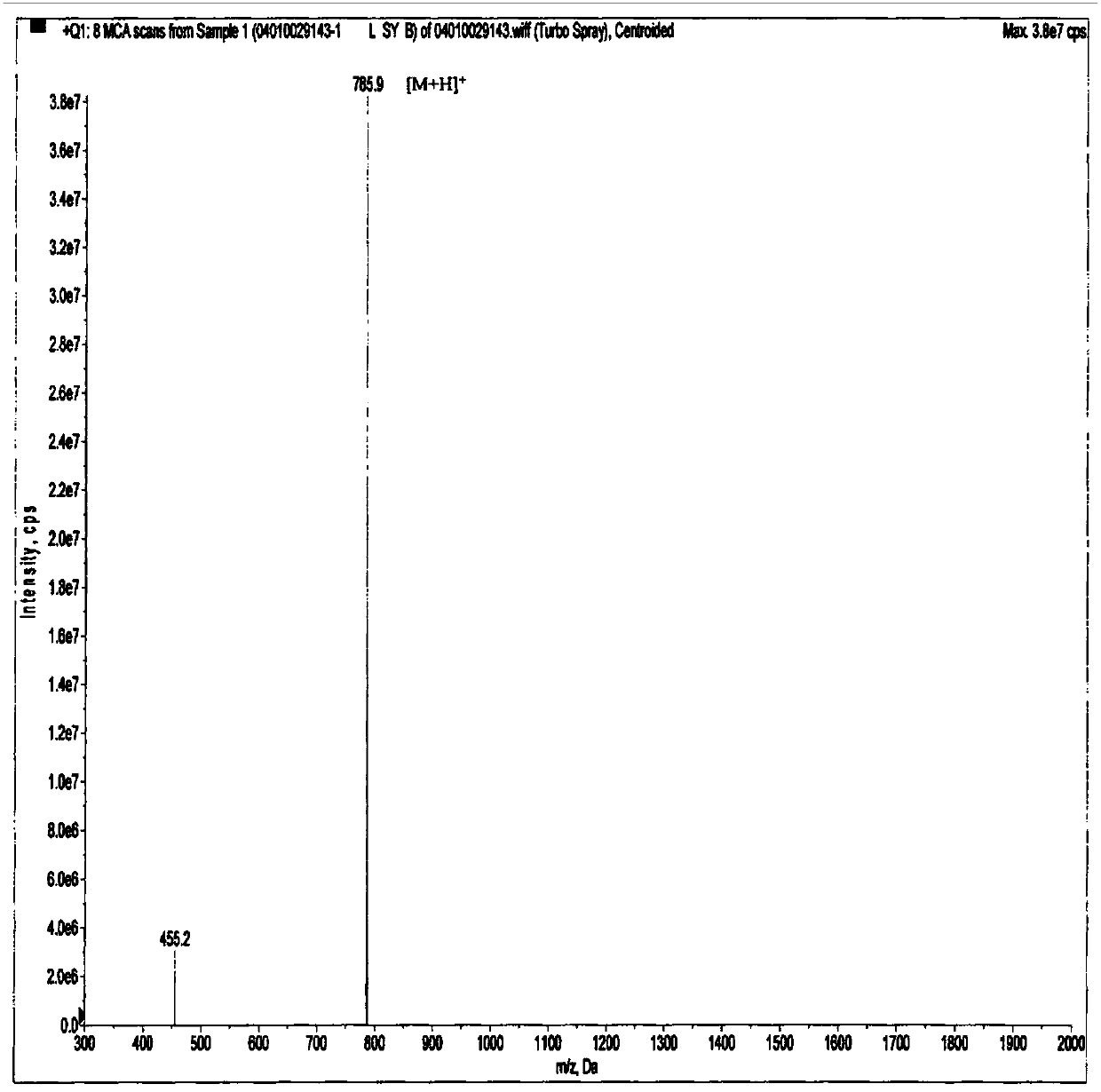
[0076] Example 1: Panning, amplification, purification, sequencing and synthesis of GPR1 antagonistic polypeptide LRH7-G6.
[0077] This example is mainly for the purpose of screening positive phages that specifically bind to GPR1, and then by amplifying and purifying the positive phages, extracting phage single-stranded DNA (ssDNA) for sequencing, analyzing and comparing the obtained sequences, and finally synthesizing high-purity phages. The antagonistic polypeptide LRH7-G6.
[0078] details as follows:
[0079] 1. Establishment of 293T cell line with permanent high expression of GPR1: 293T-GPR1 + / + / LRH
[0080] ①Select vigorously growing luminescent human 293T cells, and the day before transfection, use 5×10 5 cells / well, inoculated in a 6-well plate, cultured until the second day, the cell fusion degree was 60%;
[0081] ② Transfect on the second day, take one culture well of a 6-well plate as a unit, dilute 3 μg of plasmid with 200 μL of opti-MEM medium, and dilute 6...
Embodiment 2
[0099] Example 2 The GPR1 antagonistic polypeptide LRH7-G6 can effectively alleviate the inhibitory effect of chemerin on the cAMP signaling pathway.
[0100] (1) Cyclic adenosine monophosphate (cAMP) ELISA:
[0101] ① Cell plating: Wild-type 293T cells and 293T cells with high expression of GPR1 (293T GPR1 + / + ), with 5ⅹ10 5 Each well was inoculated in a 6-well cell culture plate, and the medium volume of each well was 1 mL. After being placed in an incubator and cultured for 24 hours, it was starved overnight, and LRH7-G6 polypeptides with different concentration gradients (3 μM, 0.3 μM, 0.03 μM) were added. , Fosklin (25μM) and chemerin (30nM) for 6h;
[0102] ②Sample preparation: Add 300 μL of cell lysate to each well, place at 4°C for 20 minutes, scrape and collect cells with a cell scraper, mix them upside down, centrifuge at 12,000 rpm for 10 minutes, and collect the supernatant;
[0103] ③ Determination of sample concentration: the sample concentration is determined b...
Embodiment 3
[0111] Example 3 GPR1 antagonistic polypeptide LRH7-G6 can effectively inhibit calcium (Ca 2+ ) inflow effect.
[0112] ① Cell plating: Wild-type 293T cells and 293T cells with high expression of GPR1 (293T GPR1 + / + ), with 5ⅹ10 3 Each cell / well was inoculated in a 96-well cell culture plate, the volume of medium in each well was 200 μL, placed in an incubator for 24 hours, and then starved overnight;
[0113] ②Reagent configuration: dissolve probenecid into 1mL buffer solution to prepare probenecid with a concentration of 250nM, shake well, add to fluorescent reagent for use;
[0114] ③Remove the cell culture medium, add LRH7-G6 polypeptide and chemerin (0.3nM) with different concentration gradients (30 μM, 3 μM, 0.3 μM, 0.03 μM, 0.003 μM) for 30 minutes, and then add 100 μL of the above fluorescent reagent to each well;
[0115] ④ Place at 37°C for 30 minutes, then at room temperature for 30 minutes;
[0116] ⑤Measure the fluorescence absorbance at excitation light 494nm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com