Technical method for reducing ceftazidime impurity H
A technology for ceftazidime tert-butyl ester and pyridine active ester is applied in the field of preparation of antibacterial drug ceftazidime, can solve the problems of unstable acid chloride preparation, high cost, difficult industrialization control and the like, achieves good color grade, mild and easy-to-control reaction conditions, The effect of improving product market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0042] Add 520ml of chloroform and 30ml of tert-butanol into a clean and dry four-necked reaction flask, and cool down to 0-5°C. Add 70g of 7-APCA and 105g of histidine active ester, and add triethylamine dropwise under temperature control to adjust the pH to 7.0-9.0. Control the temperature at 0-10°C and react for 20-24 hours. After the reaction is complete, add 150ml of pure water, stir for 1-2 hours, and separate the chloroform. Dropping isopropanol into the liquid medicine to precipitate ceftazidime tert-butyl ester, stirring and growing crystals at 0-10°C for 2 hours. Filter with suction, wash with isopropanol, and dry to obtain the dry product of ceftazidime tert-butyl ester.
[0043] Weight yield: 145%, purity 98.9%,
Embodiment 1-2
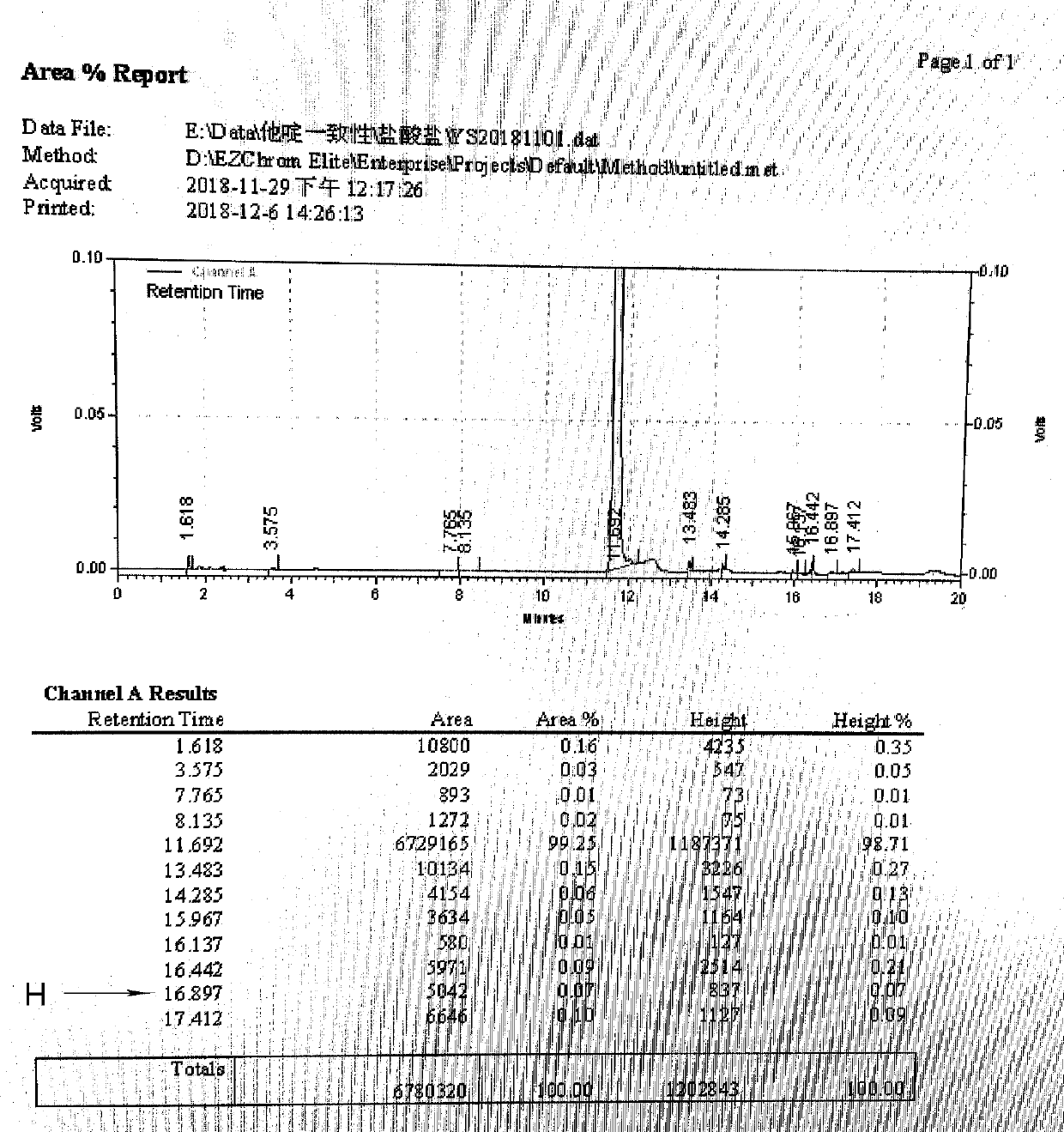
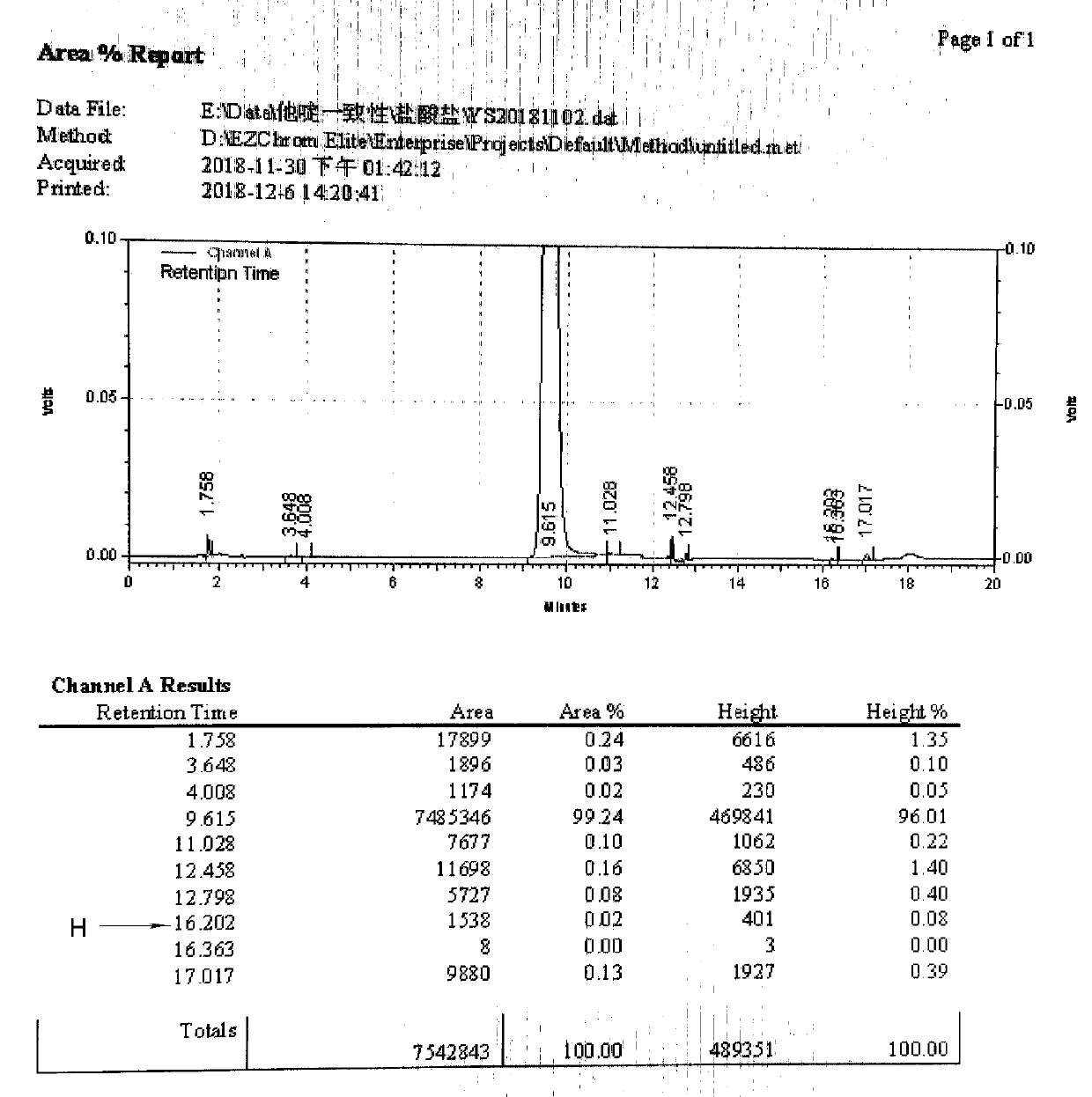
[0045] Add 54ml of formic acid and 36ml of concentrated hydrochloric acid into a clean and dry four-necked reaction flask, and stir to mix. Add 60 g of ceftazidime tert-butyl ester, react at 15-20° C. for 3 hours, and add acetone dropwise at room temperature until ceftazidime hydrochloride is completely precipitated. 0-10°C crystal growth for 2 hours. Suction filtration, washing with acetone, and drying to obtain dry product of ceftazidime hydrochloride.
[0046] Weight yield: 88%, purity 99.3%, H: 0.07%.
Embodiment 1-3
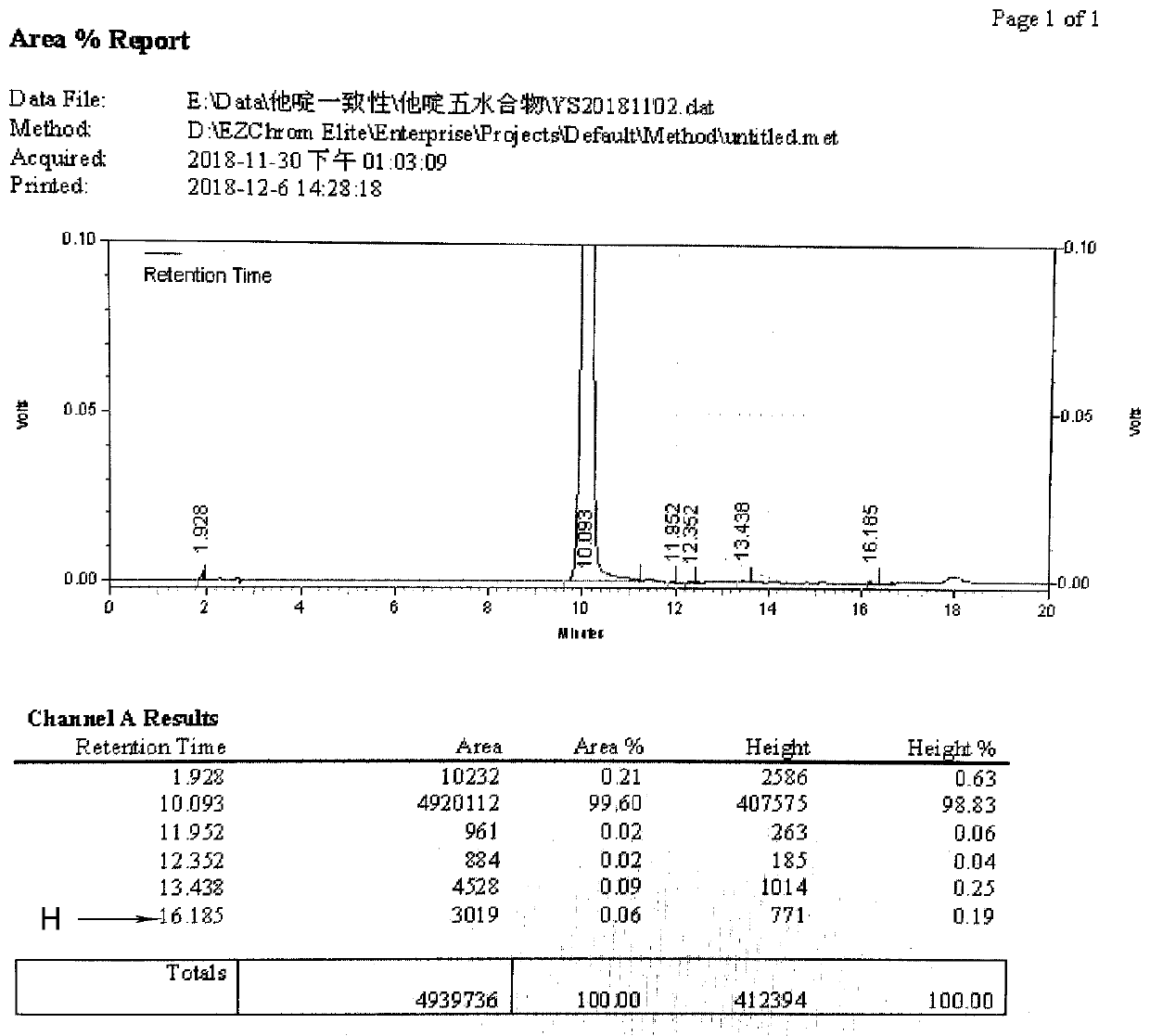
[0048]Add 20ml of water for injection and 30ml of acetone into a clean and dry four-necked reaction bottle, and stir to mix. Add 50 g of ceftazidime hydrochloride, add dropwise 4N NaOH, and adjust the pH to 6.0-6.5 to dissolve it. After dissolving, add 1 g of activated carbon and decolorize for 10 minutes. Filtration, the filtrate was added dropwise with 4M H 3 PO 4 , until the crystals were completely precipitated. 0-10 ℃ stirring crystal growth for 1 hour. Filter with suction, wash with acetone, and dry to obtain the dry product of ceftazidime pentahydrate.
[0049] Weight yield: 80%, purity 99.6%, H: 0.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com