Solid forms of adamantyl compound, compositions and uses thereof
A compound and composition technology, applied in the field of preparation of medicines for treating central nervous system diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1. ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methylbenzoate (compound (I)) preparation of
[0180]
[0181] In a 100mL single-necked bottle, add ((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)chloromethyl carbamate (2.0g, 7.35mmol), benzoic acid (0.98 g, 8.09mmol), triethylamine (0.89g, 8.82mmol), sodium iodide (0.55g, 3.67mmol) and N,N-dimethylformamide (8mL), heated to 85°C. After reacting for 2 h, samples were taken for TLC detection. After the reaction, cool down to room temperature, add water (30mL) and ethyl acetate (30mL); after stirring at room temperature for 30min, static layering; separate the organic layer, and wash the organic layer once with saturated aqueous sodium bicarbonate (30mL) , washed once with HCl (0.5M, 30mL), and finally washed once with water (30mL); the organic layer was separated, and ethyl acetate was distilled off under reduced pressure to obtain a brown oil (1.2g); a white solid was obtained after column chromatogr...
Embodiment 2
[0185] Example 2. ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methylbenzoate (compound (I)) In vivo pharmacokinetic analysis
[0186] In the experiment, the in vivo conversion of compound (I) to the active compound memantine was tested.
[0187] Materials and methods
[0188] LC / MS / MS analysis system: Agilent 1200 series vacuum degasser, binary syringe pump, orifice autosampler, column oven, charged spray ionization (ESI) source and Agilent G6430 triple quadrupole mass spectrometer. Quantitative analysis was performed in MRM mode, and the MRM conversion parameters were as follows:
[0189] multiple reaction detection scan
180.2→163.1
Fragmentation voltage
15V
capillary voltage
3500V
Dryer temperature
350℃
atomizer
40psi
Dryer flow rate
9L / min
[0190] A Waters XBridge TMC 18, 2.1 x 30 mm, 3.5 μM column was used to inject 20 μL of sample each time. Analysis conditions: the mobile phase includ...
Embodiment 3
[0199] Example 3. Preparation of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methylbenzoate Form I
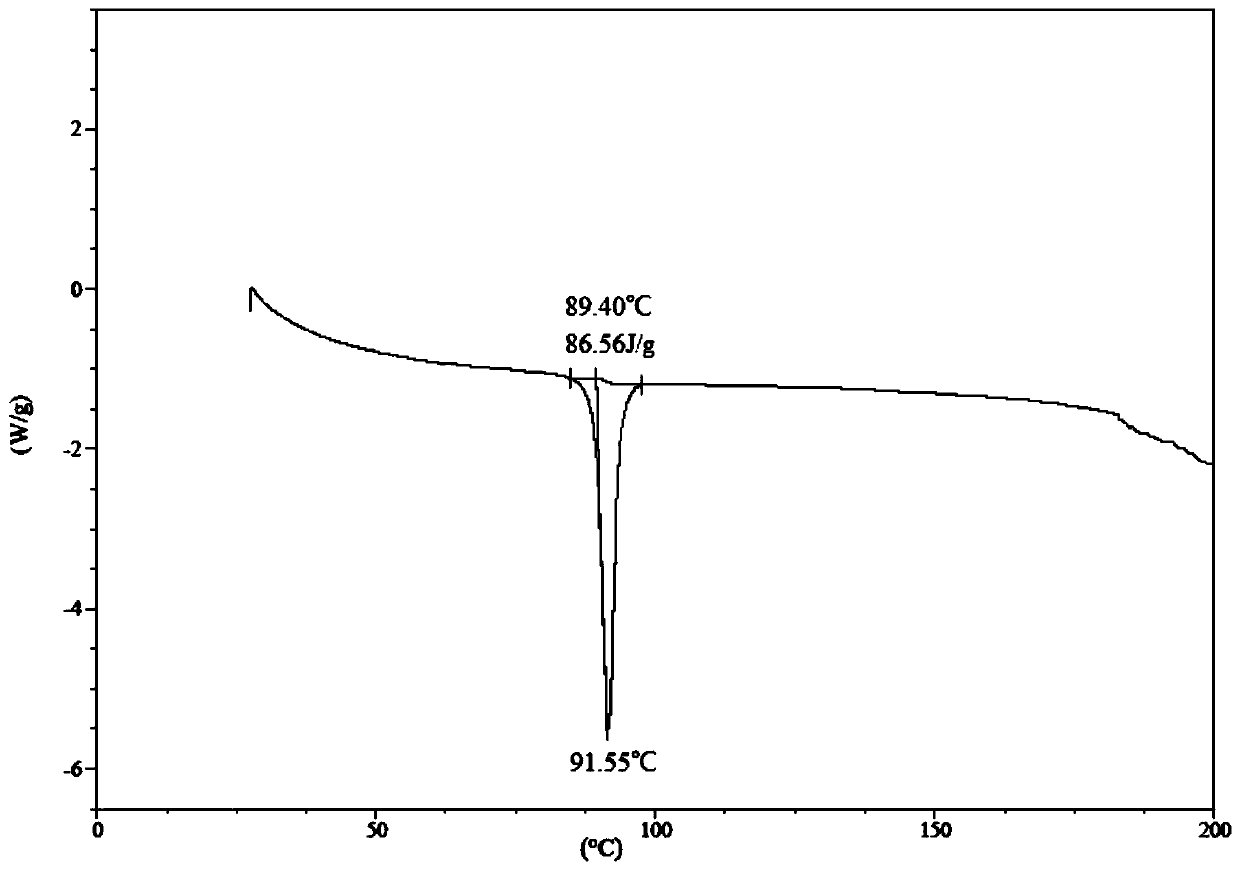
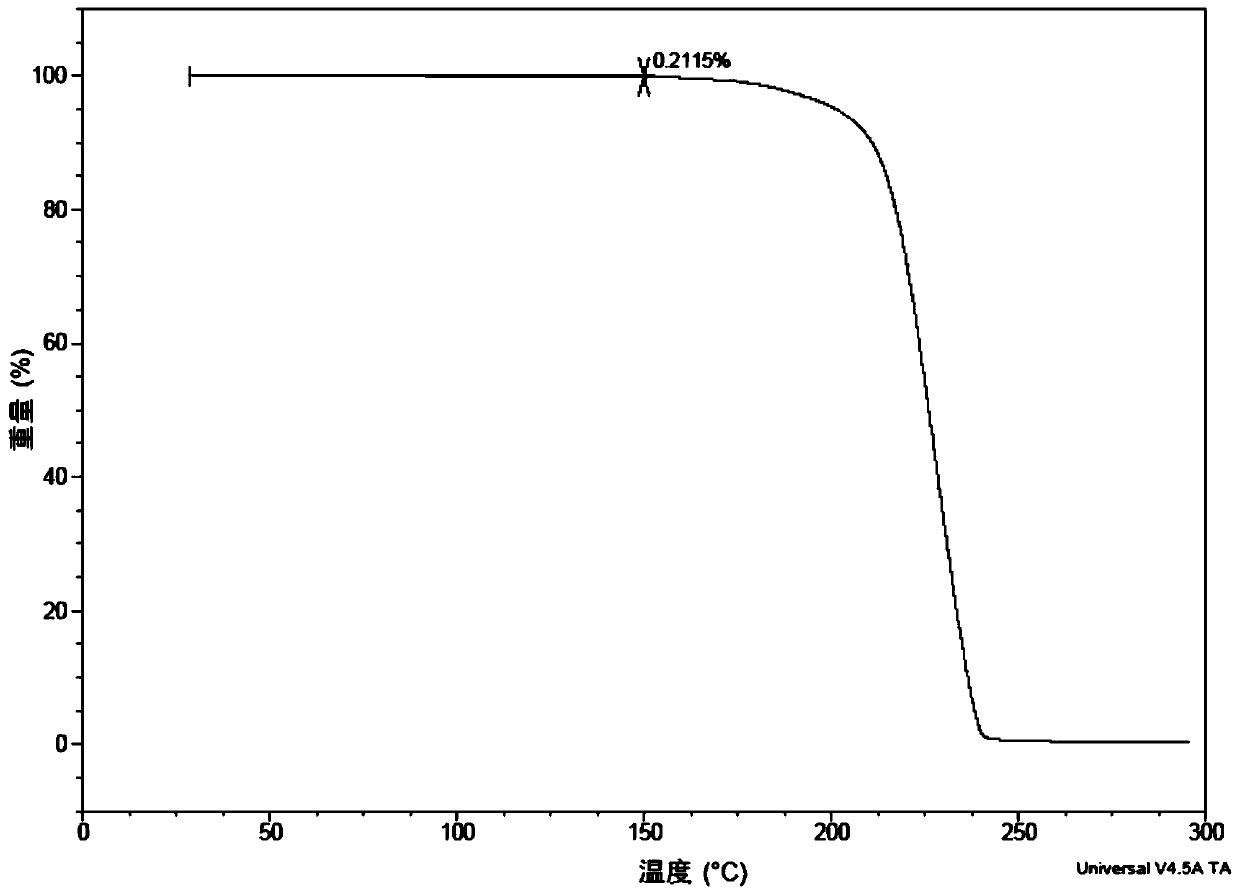
[0200] Crude ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methylbenzoate (2.00 g) was added to dry iso in propanol (20mL); placed in a 40°C oil bath and stirred to obtain a clear solution, then slowly cooled to room temperature, and then slowly added water (20mL); a white solid precipitated, suction filtered, and the filter cake was placed in a drying oven at room temperature under vacuum After drying, white crystals (1.88 g, yield 94%) were obtained, which were confirmed to be Form I by XPRD and DSC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com