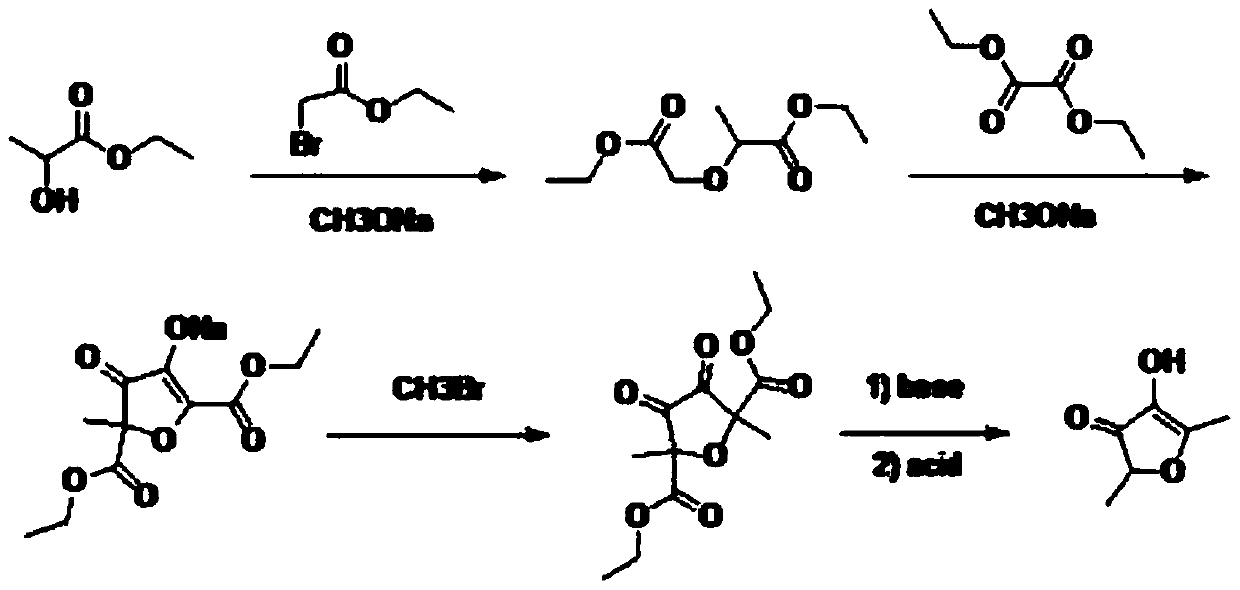
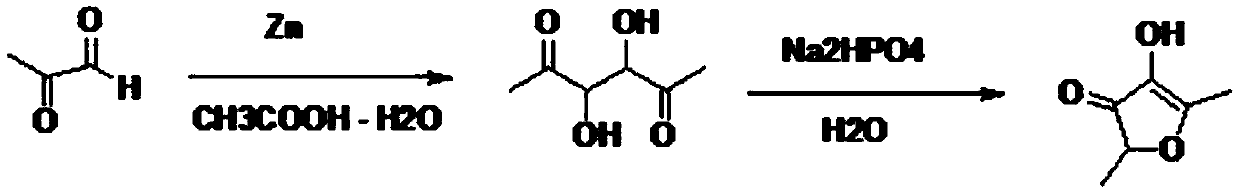
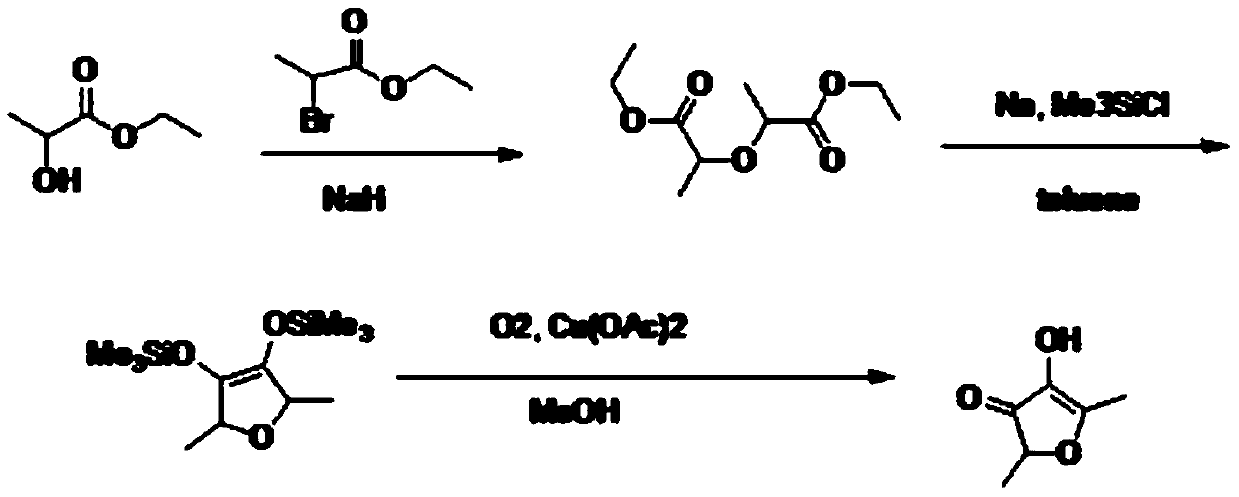
Preparation method of 2,5-dimethyl-4-hydroxyl-3(2H)furanone
A technology of diacetylfuran and diacetoxyfuran, which is applied in the field of preparation of 2,5-dimethyl-4-hydroxy-3-furanone, can solve the problems of lack of green chemical process and achieve synthesis operation and purification The effect of simplicity, high yield, and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of the present embodiment 2,5-dimethyl-3,4-diacetylfuran is as follows:
[0038] (1) Preparation of 2,5-dimethyl-3,4-diacetylfuran
[0039]The reaction device is: a 2000ml four-neck round bottom flask equipped with a thermometer, a mechanical stirrer, a water separator, a condenser tube and a silicone oil bath. The four-necked round-bottomed flask is placed in a silicone oil bath, and the thermometer and the water separator are installed on the four-necked round-bottomed flask, and the condenser tube is installed on the upper end of the water separator, and 198g of 3, 4-Diacetyl-2,5-hexanedione, 1000ml of xylene and 6.8g of potassium hydrogensulfate, start the stirrer to stir, heat up the silicone oil bath and reflux to separate the water, stop heating until no water comes out, cool to room temperature, filter The catalyst was removed, and the solvent was removed from the filtrate by distillation under reduced pressure to obtain 190.7 g of crude ...
Embodiment 2
[0045] The preparation method of the present embodiment 2,5-dimethyl-3,4-diacetylfuran is as follows:
[0046] (1) Preparation of 2,5-dimethyl-3,4-diacetylfuran
[0047] The reaction device is: a 2000ml four-neck round bottom flask equipped with a thermometer, a mechanical stirrer, a water separator, a condenser tube and a silicone oil bath. Place the four-necked round-bottomed flask in a silicone oil bath, install the thermometer and the water separator on the four-necked round-bottomed flask, and install the condenser tube on the upper end of the water separator, add 198g of 3, 4-Diacetyl-2,5-hexanedione, 1000ml of toluene and 15g of solid sulfonic acid (high-temperature type, produced by Kairui Environmental Technology Co., Ltd.), start stirring, raise the temperature and reflux to separate water until almost no water comes out and stop heating , cooled to room temperature, filtered off the catalyst solid sulfonic acid, and the filtrate was distilled under reduced pressure...
Embodiment 3
[0053] The preparation method of the present embodiment 2,5-dimethyl-3,4-diacetylfuran is as follows:
[0054] (1) Preparation of 2,5-dimethyl-3,4-diacetylfuran
[0055] The reaction device is: a 2000ml four-neck round bottom flask equipped with a thermometer, a mechanical stirrer, a water separator, a condenser tube and a silicone oil bath. The four-necked round-bottomed flask is placed in a silicone oil bath, and the thermometer and the water separator are installed on the four-necked round-bottomed flask, and the condenser tube is installed on the upper end of the water separator, and 198g of 3, 4-Diacetyl-2,5-hexanedione, 1000ml of xylene and 10.8g of solid phosphonic acid, start the stirrer to stir, heat up the silicone oil bath and reflux to separate water, stop heating until no water comes out, cool to room temperature, filter The catalyst was removed, and the solvent was removed from the filtrate by distillation under reduced pressure to obtain 188.6 g of crude 2,5-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com