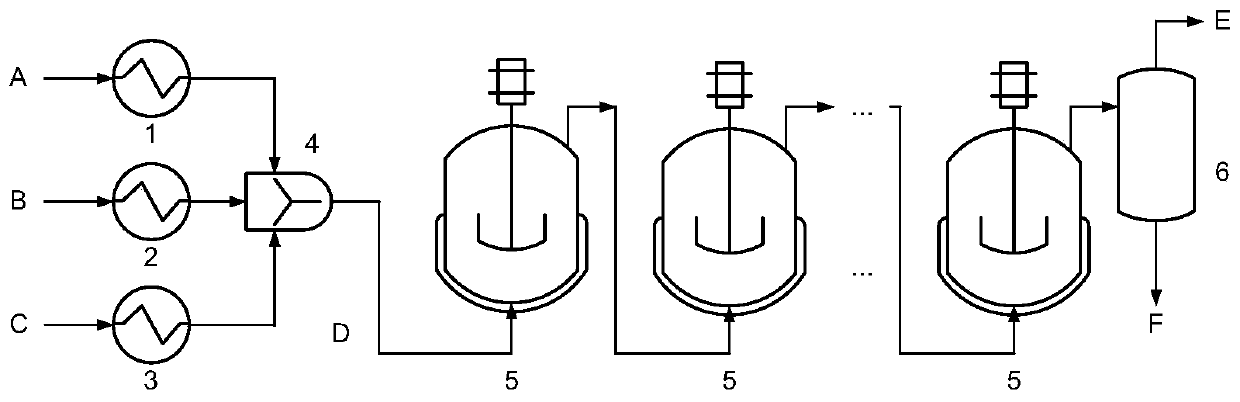
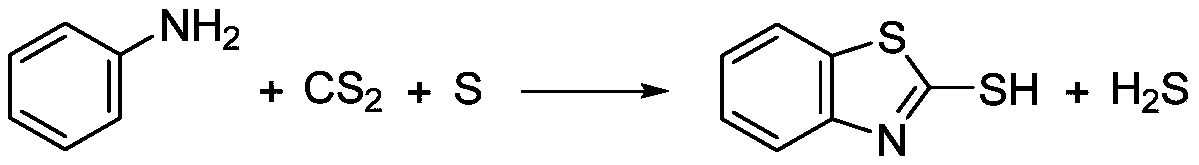
Reaction device for continuous synthesis of 2-mercaptobenzothiazole
A technology of mercaptobenzothiazole and reactor, which is applied in the direction of organic chemistry, can solve problems such as fire, difficulty in controlling the formation of benzothiazole and anilinobenzothiazole, and lack of rapid mixing ability, so as to achieve process safety and product stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The molar ratio of aniline, carbon disulfide and sulfur is 1.0:1.1:1.0. At room temperature, it is preheated to 150°C, 40°C, and 180°C respectively through a metering pump and heat exchanger at room temperature, and then enters the micro-mesh dispersing mixer (inner Contains 8 cross-flow shearing screens, the apertures are all 0.5mm, 4 of which are used to disperse sulfur in aniline, and 4 are used to disperse carbon disulfide in aniline), and react in 3-stage series stirred tanks (reaction tank The temperature is 260°C, 250°C and 210°C respectively), the reaction pressure is 8MPa, and the residence time is 1.5h. After hydrogen sulfide is separated by gas stripping (the stripper temperature is 210°C and the pressure is 8MPa), the high-pressure product of 2-mercaptobenzothiazole with a purity of 92% is obtained, and the finished product 2-mercaptobenzothiazole is obtained through alkali-soluble filtration and drying. The melting point is 175°C.
Embodiment 2
[0023] The molar ratio of aniline, carbon disulfide, and sulfur is 1.0:1.2:1.05. They are preheated to 170°C, 40°C, and 150°C through metering pumps and heat exchangers at room temperature, and then enter the micro-mesh dispersing mixer (inner Contains 2 cross-flow shearing screens, the apertures are 2mm, one of which is used to disperse aniline in carbon disulfide, and the other is used to disperse sulfur in carbon disulfide), react in 4-stage series stirred tank (the temperature of the reaction tank 270°C, 260°C, 250°C and 210°C respectively), reaction pressure 8MPa, residence time 2h. After hydrogen sulfide is separated by gas stripping (stripping tower temperature 210°C, pressure 8MPa), the high-pressure product of 2-mercaptobenzothiazole with a purity of 94% is obtained, and the finished product 2-mercaptobenzothiazole is obtained by filtering and drying with alkali. The melting point is 174°C.
Embodiment 3
[0025] The molar ratio of aniline, carbon disulfide, and sulfur is 1.0:1.1:1.1. At room temperature, it is preheated to 200°C, 60°C, and 120°C through a metering pump at room temperature through a heat exchanger, and then enters a micro-channel mixer (a cross-shaped Microchannel structure, each of the three raw materials enters from a branch channel, and the last channel is used as the outlet of the mixed product, the channel hydraulic diameter is 5mm), and the reaction is carried out in a 4-stage series stirred tank (the temperature of the reactor is 270 ° C, 260 ° C, 250 ° C, respectively. ℃ and 210℃), the reaction pressure is 9MPa, and the residence time is 2.3h. After hydrogen sulfide is separated by gas stripping (stripping tower temperature 210°C, pressure 9MPa), the high-pressure product of 2-mercaptobenzothiazole with a purity of 95% is obtained, and the finished product 2-mercaptobenzothiazole is obtained by filtering and drying with alkali. The melting point is 176°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com