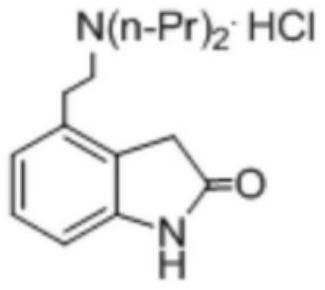
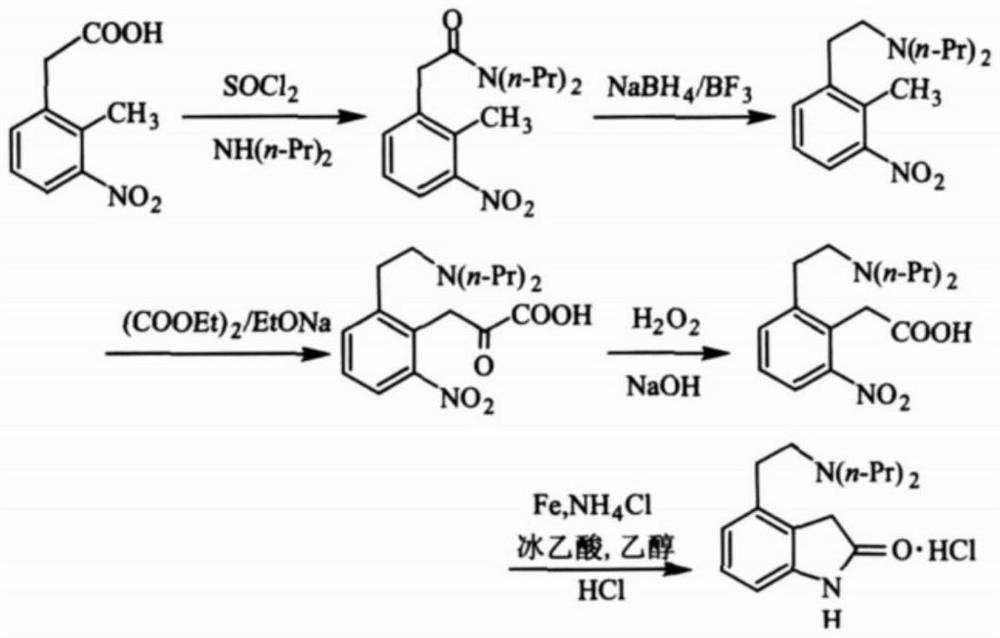
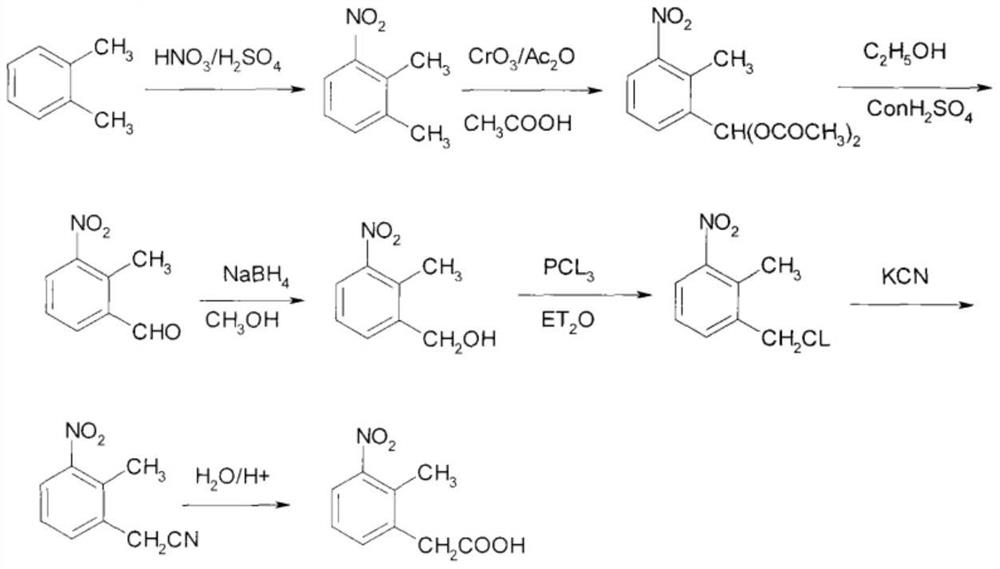
The synthetic method of ropinirole hydrochloride intermediate 2-methyl-3-nitrophenylacetic acid
A technology for ropinirole hydrochloride and nitrophenylacetic acid, which is applied in the field of synthesis of ropinirole hydrochloride intermediate 2-methyl-3-nitrophenylacetic acid, can solve drainage pollution, low overall yield and pollution Large and other problems, to achieve the effect of less pollution discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthetic method of 2-methyl-3-nitrophenylacetic acid comprises the following steps:
[0033] 1) Under argon protection, mix ethyl iodoacetate, magnesium ethoxide, catalyst and anhydrous tetrahydrofuran, control the reaction temperature to rise to 112°C, and the pressure to 4.5 atmospheres, dropwise add 6-iodo-2- The mixture composed of nitrotoluene and anhydrous isopropanol is controlled to be added for 50 minutes. After the addition is completed, the temperature is controlled to rise to 140°C and the pressure is raised to 8 atmospheres. After continuing to react for 3 hours, cool to room temperature and drop Add a 12% sodium hydroxide aqueous solution, and control the temperature not to exceed 90°C. After the sodium hydroxide aqueous solution is added dropwise, control the temperature to rise to 132°C, and the pressure is 2.6 atmospheres, and the reaction is completed for 1.5 hours.
[0034] The preparation method of the catalyst is as follows: the nanometer molec...
Embodiment 2
[0038] The synthetic method of 2-methyl-3-nitrophenylacetic acid comprises the following steps:
[0039] 1) Under nitrogen protection, mix ethyl bromoacetate, magnesium ethoxide, catalyst and absolute ethanol, control the reaction temperature to rise to 100°C, and the pressure to 3 atmospheres, dropwise add 6-chloro-2-nitrate The mixture composed of methyl toluene and anhydrous isopropanol is controlled to be added for 30 minutes. After the addition is completed, the temperature is increased to 130°C and the pressure is increased to 7 atmospheres. After continuing the reaction for 2 hours, cool to room temperature and add dropwise The mass fraction of potassium hydroxide aqueous solution is 12%, and the temperature is controlled not to exceed 90°C. After the potassium hydroxide aqueous solution is added dropwise, the temperature is controlled to rise to 120°C, the pressure is 2 atmospheres, and the reaction is completed for 1 hour.
[0040] The preparation method of the cataly...
Embodiment 3
[0044] The synthetic method of 2-methyl-3-nitrophenylacetic acid comprises the following steps:
[0045]1) Under argon protection, mix ethyl iodoacetate, magnesium ethoxide, catalyst and anhydrous tetrahydrofuran, control the reaction temperature to rise to 120°C, and the pressure to 5 atmospheres, dropwise add 6-bromo-2- The mixture composed of nitrotoluene and anhydrous isopropanol is controlled to drop for 60 minutes. After the drop is completed, the temperature is controlled to rise to 155 ° C, and the pressure is raised to 9 atmospheres. After continuing to react for 4 hours, cool to room temperature and drop Add 12% sodium hydroxide aqueous solution, control the temperature not to exceed 90°C, after the sodium hydroxide aqueous solution is added dropwise, control the temperature to rise to 140°C, and the pressure is 3 atmospheres, and the reaction is completed for 2 hours.
[0046] The preparation method of the catalyst is as follows: the nanometer molecular sieve is soa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com