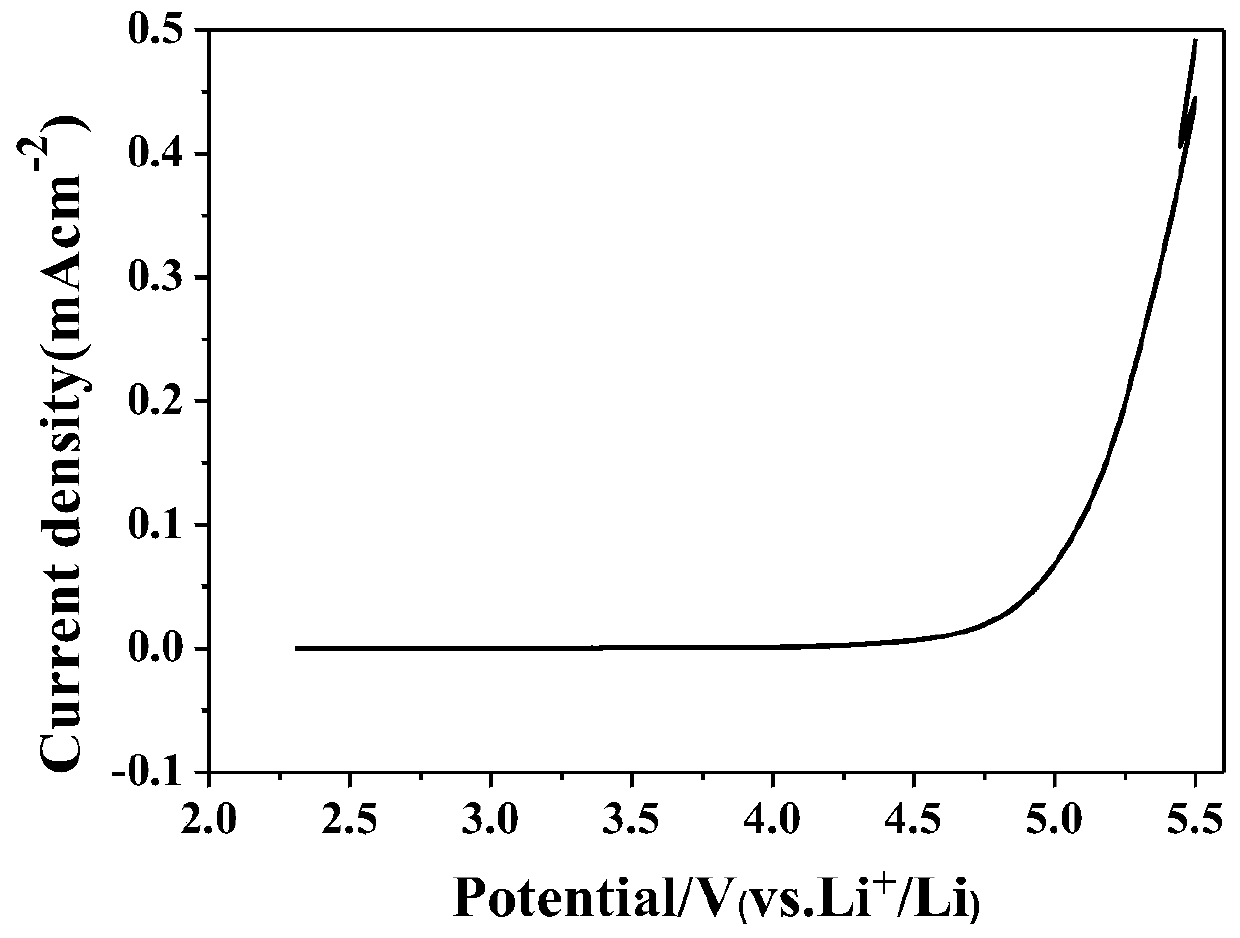
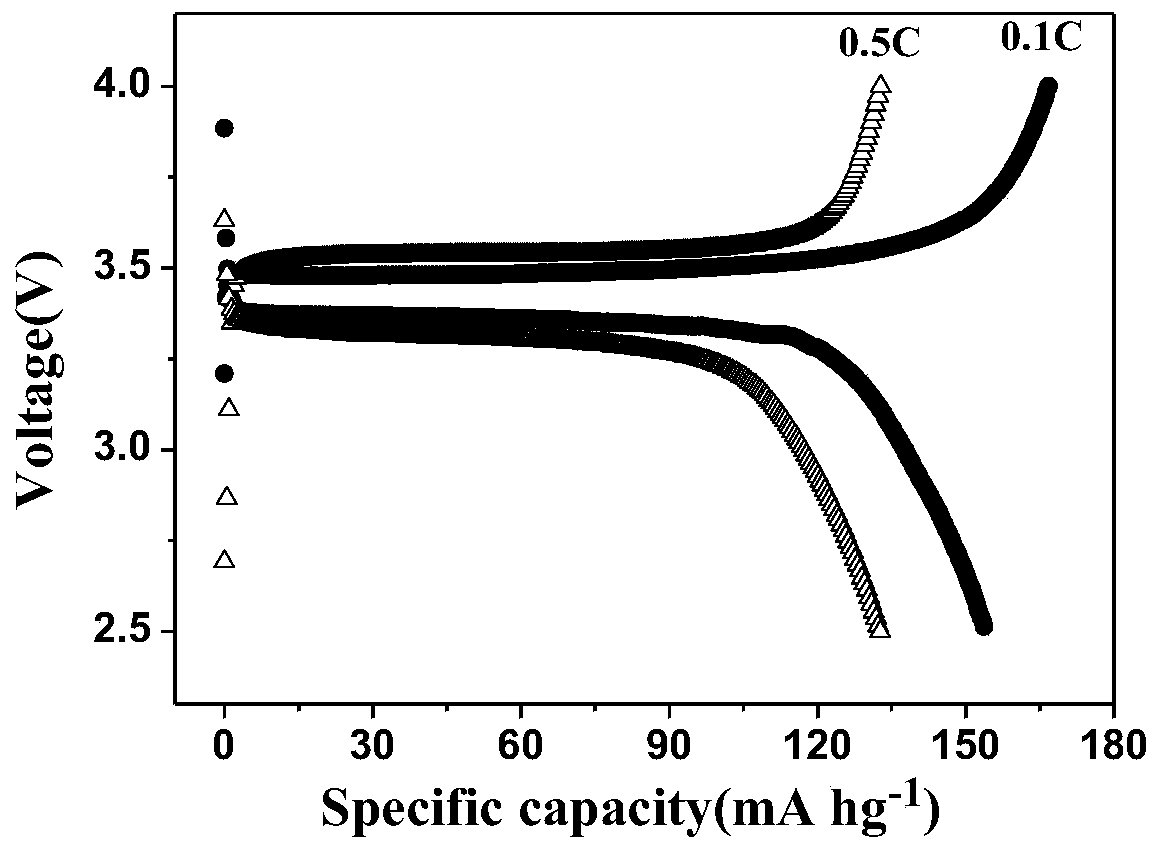
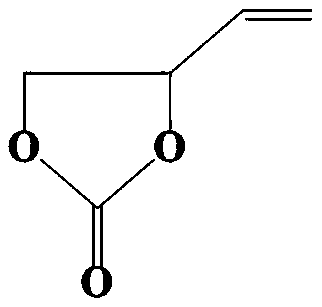
Preparation method and application of polycarbonate-based polymer electrolyte
A polycarbonate and polymer technology, used in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of low electrochemical window and unsuitable high-nickel cathode material system, so as to eliminate environmental pollution and improve long-term performance. Cyclic stability performance, damage suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 3 g of ethylene carbonate and 0.8 g of lithium bistrifluoromethanesulfonimide (LiTFSI) in 5 ml of acetonitrile, stir at room temperature to dissolve completely; add 0.1 g of azobisisobutyronitrile and stir evenly. On the polytetrafluoroethylene mold, using whatman membrane as a porous support framework, scrape-coat the evenly stirred mixture on both sides of whatman membrane; heat at 80°C for 10 hours in a vacuum oven to solidify to form a film.
Embodiment 2
[0031] Dissolve 1g of ethylene carbonate and 0.25g of lithium bistrifluoromethanesulfonylimide (LiTFSI) into 1.5ml of N-methylpyrrolidone (NMP), stir at room temperature to dissolve completely; add 0.02g of azobis Isobutyronitrile was stirred evenly. On the polytetrafluoroethylene mold, using whatman membrane as a porous support framework, scrape-coat the evenly stirred mixture on both sides of whatman membrane; heat at 80°C for 10 hours in a vacuum oven to solidify to form a film.
Embodiment 3
[0033] Dissolve 1.38g of ethylene carbonate and 0.4g of lithium bistrifluoromethanesulfonylimide (LiTFSI) into 1.5ml of N-methylpyrrolidone (NMP), stir at room temperature to dissolve completely; add 0.02g of bis( acetylacetonate) dibutyltin and stir evenly. On the polytetrafluoroethylene mold, using whatman membrane as a porous support framework, scrape-coat the evenly stirred mixture on both sides of whatman membrane; heat at 80°C for 10 hours in a vacuum oven to solidify to form a film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com