Crosslinking hyaluronic acid gel resisting hydrolysis of hyaluronidase
A technology for cross-linking hyaluronic acid and hyaluronidase, applied in the field of materials, can solve problems such as adverse reactions, and achieve the effects of large application potential, large economic benefits, and enhanced maintenance time and plasticity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
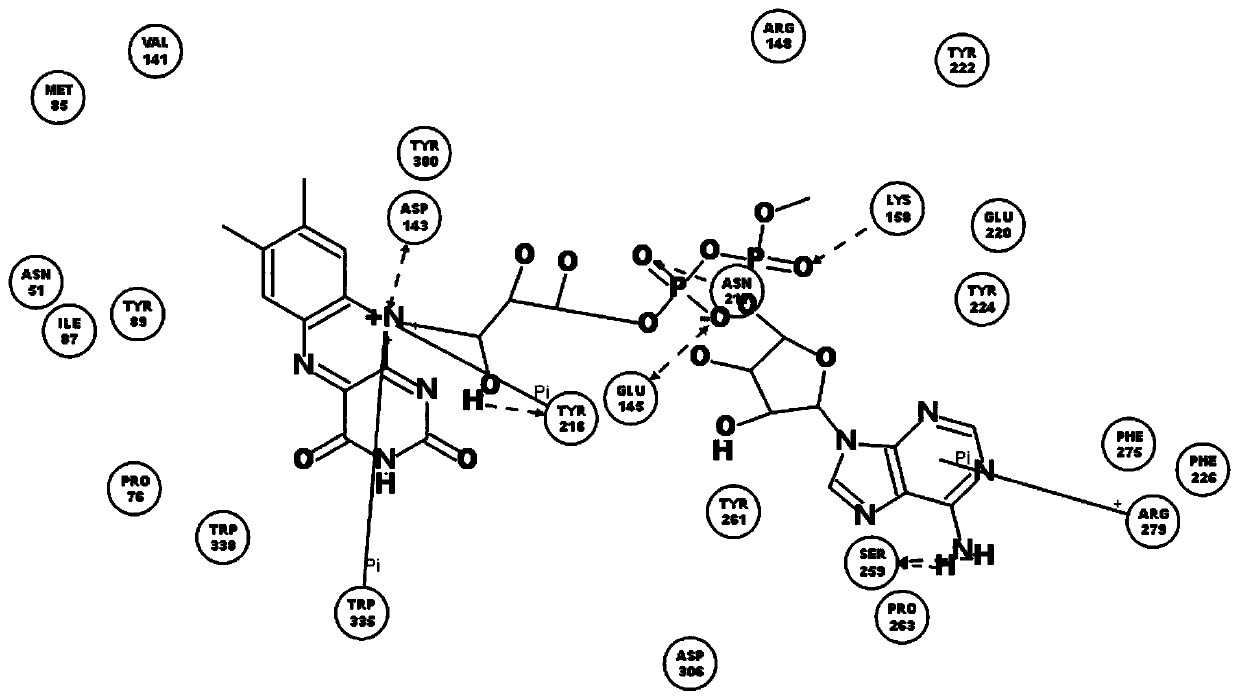
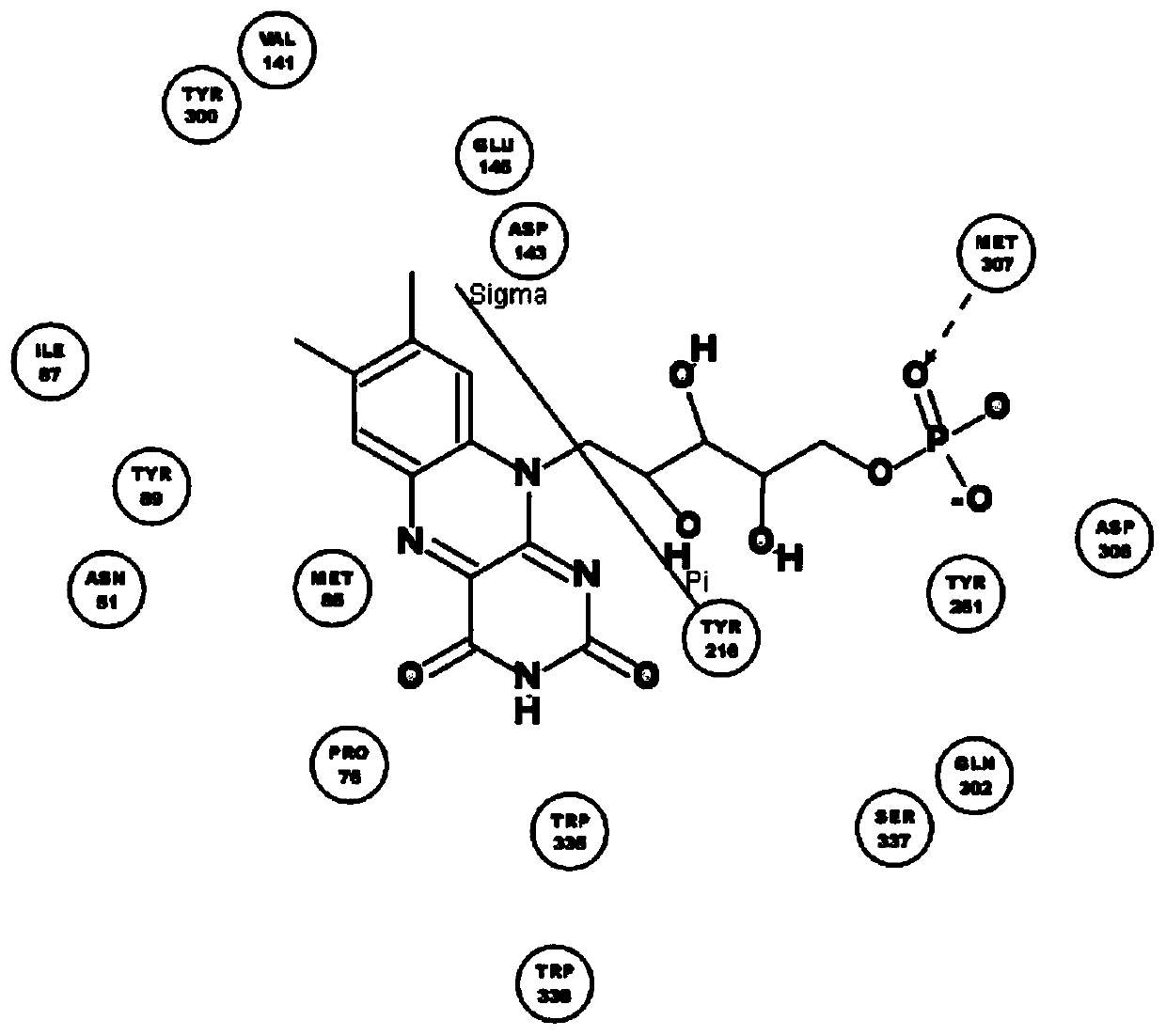
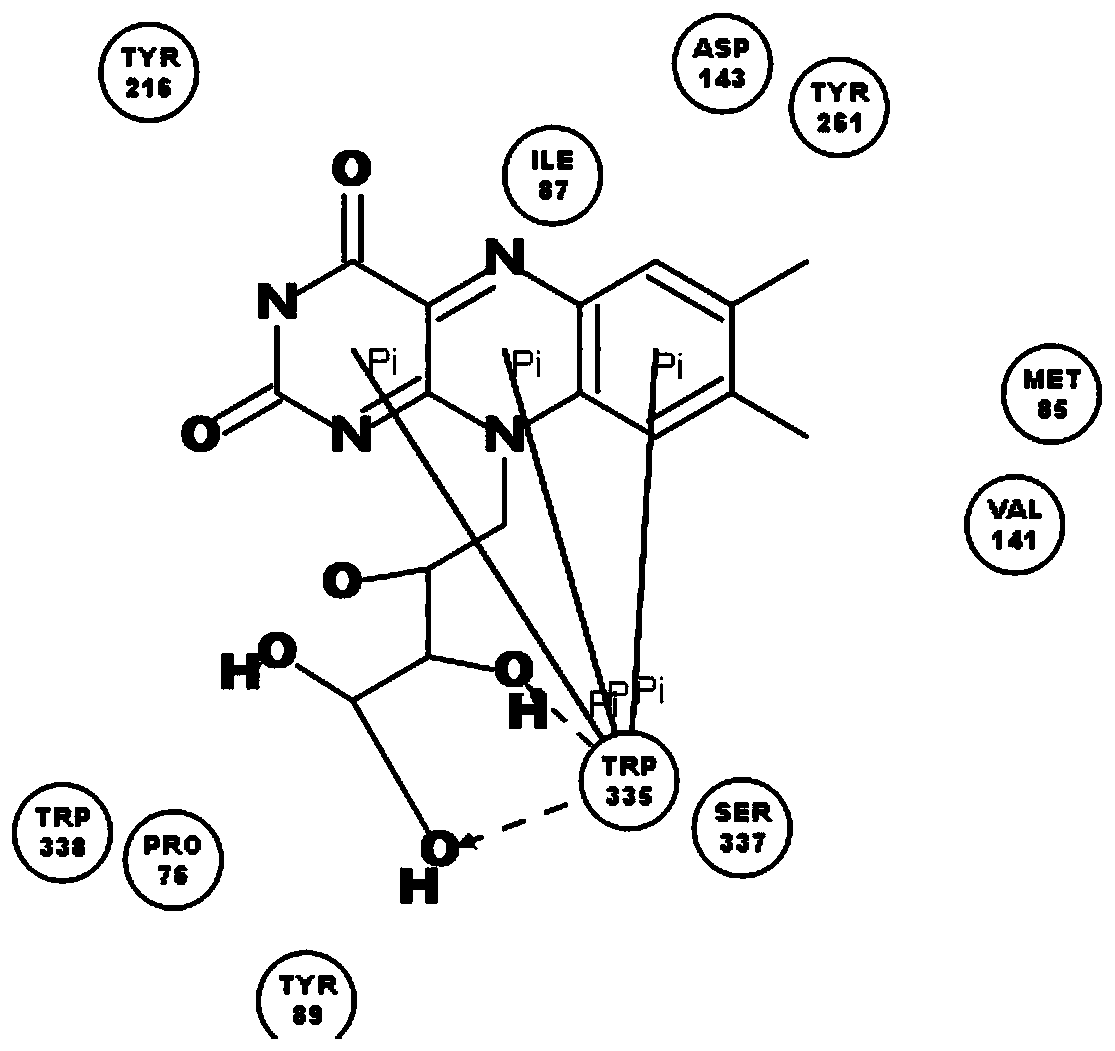
[0038] The names of natural flavin coenzymes and their derivatives are: including riboflavin: Riboflavin, isoalloxazine: isoalloxazine,
[0039] 7-methyl-8-chloro-10-(1′-D-ribityl)isoalloxazine(7-M-8-C),
[0040] 7-bromo-8-methyl-10-(1′-D-ribityl)isoalloxazine(7-B-8-M),
[0041] 7-methyl-8-bromo-10-(1′-D-ribityl)isoalloxazine(7-M-8-B),
[0042] 7-chloro-8-ethyl-10-(1′-D-ribityl)isoalloxazine(7-C-8-E),
[0043] 7,8-diethyl-10-(1′-D-ribityl)isoalloxazine (7,8-D).
Embodiment 2
[0046] Inhibition of human recombinant hyaluronidase activity by natural flavin coenzyme and its derivatives
[0047] The establishment of the reaction system:
[0048] 0.5g of cross-linked hyaluronic acid gel, the content of hyaluronic acid is 28mg / g, add 2mL of PBS buffer solution, pH 6.5-7.5, which contains 250U / mL of human-derived recombinant hyaluronidase; in addition, add a certain amount of Natural flavin coenzyme or its derivative molecule. The entire reaction system was placed in a water bath at 37° C. for 72 hours, samples were taken every 24 hours, centrifuged at 12,000 rpm for 10 minutes, and then the supernatant was removed to determine the content of free hyaluronic acid.
[0049] (1) Determination of the optimal addition amount of FAD
[0050] Determine the maximum amount of ligand molecule added. In the above reaction system, add 0, 0.2, 0.4, 0.6, 0.8, 1.0mmol / L of FAD, in a water bath at 37°C for 72 hours, sample every 24 hours, centrifuge at 12000rpm for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com