Oxide ceramic composite solid electrolyte and preparation method and application thereof
A technology of oxide ceramics and solid electrolytes, which is applied in the field of electrochemistry, can solve problems such as deterioration, difficulty in compensating the volume change of electrode material deintercalation lithium, and safety issues, so as to inhibit the formation of lithium dendrites and improve the interface between electrolyte and electrode Good contact and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
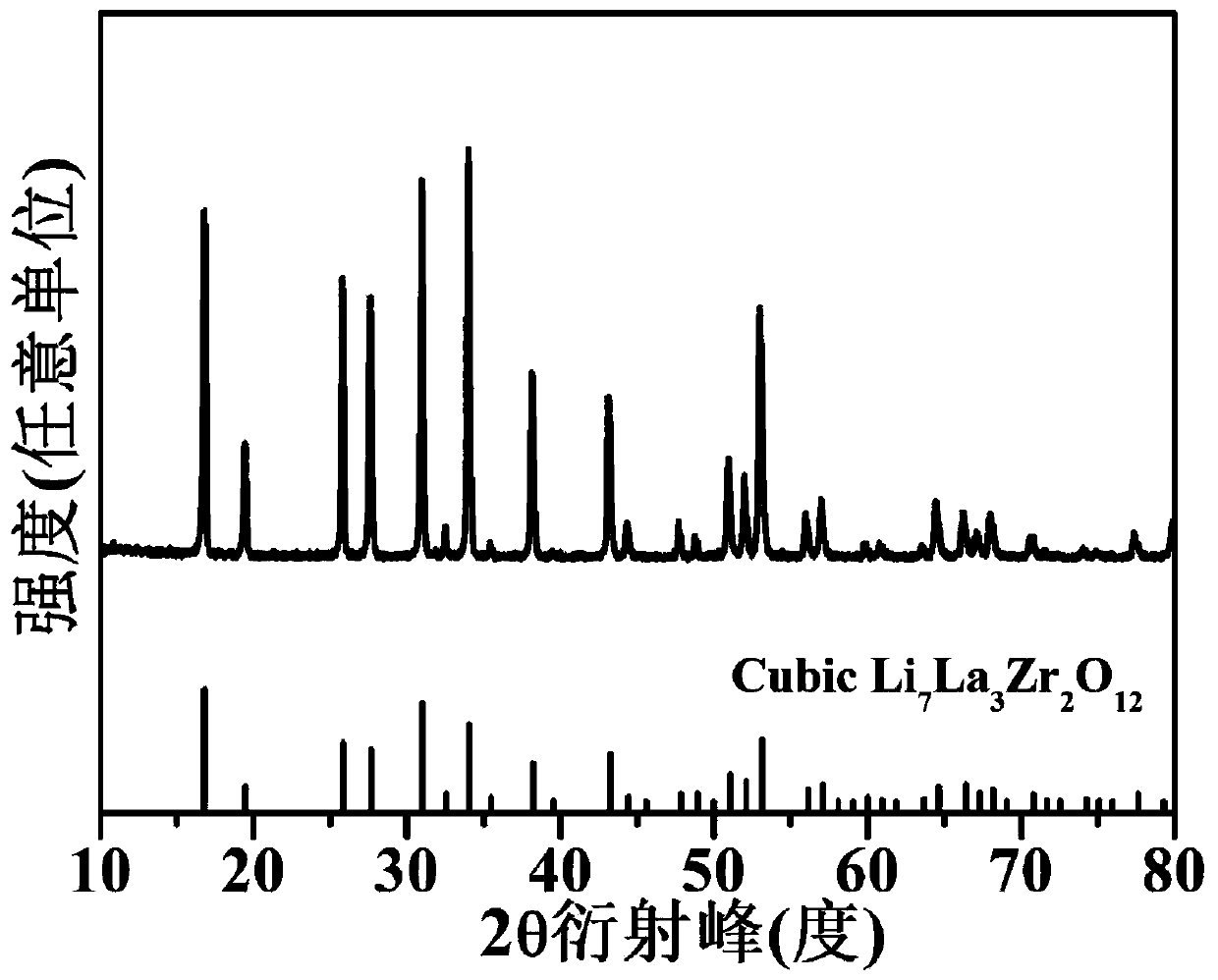
[0029] Preparation of garnet-type ceramic electrolyte Li by traditional solid-state sintering method 6.4 La 3 Zr 1.4 Ta 0.6 o 12 . Weigh Li according to the stoichiometric ratio 2 CO 3 , La 2 o 3 , ZrO 2 and Ta 2 o 5 , and 30ml of isopropanol were added into the ball mill jar, and ball milled at a speed of 250rpm for 20h. In order to compensate for the loss of lithium volatilization during high-temperature calcination, set Li 2 CO3 10% excess. After the ball milling, the obtained mixed solution was dried in a blast oven at 80° C. for 12 hours to obtain a precursor powder. The obtained powder was transferred to a porcelain boat and calcined in a tube furnace at 900 °C for 8 h in an air atmosphere. The pre-sintered powder was ground again, and then calcined at 1100 °C in air for 24 h to obtain cubic Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 .
[0030] Weigh 0.8 g of polyethylene oxide and 0.8 g of polypropylene carbonate and add them into 50 ml of anhydrous acetonitrile...
Embodiment 2
[0035] Preparation of garnet-type ceramic electrolyte Li by traditional solid-state sintering method 6.2 Al 0.2 La 3 Zr 1.8 Ta 0.2 o 12 . Weigh LiOH, Al according to stoichiometric ratio 2 o 3 , La 2 o 3 , ZrO 2 and Ta 2 o 5 , and 50ml of isopropanol were added into a ball mill jar, and ball milled at a speed of 300rpm for 24h. In order to compensate for the loss of lithium volatilization during high-temperature calcination, an excess of 15% LiOH is set. After the ball milling, the obtained mixture was dried in a blast oven at 60° C. for 8 hours to obtain a precursor powder. The resulting powder was transferred to a porcelain boat and calcined at 800 °C for 6 h in an air atmosphere in a tube furnace. The pre-sintered powder was ground again, and then calcined at 1050 °C in air for 20 h to obtain cubic Li 6.2 Al 0.2 La 3 Zr 1.8 Ta 0.2 o 12 .
[0036] Weigh 1.4 g of polyethylene oxide and 0.2 g of polypropylene carbonate and add them into 50 ml of N-methylpy...
Embodiment 3
[0041] Preparation of garnet-type ceramic electrolyte Li by traditional solid-state sintering method 6.35 La 2.95 Rb 0.05 Zr 1.2 Ta 0.8 o 12 . Weigh LiOH·H according to the stoichiometric ratio 2 O, La 2 o 3 , Rb 2 CO 3 , ZrO 2 and Ta 2 o 5 , and 50ml of isopropanol were added into a ball mill jar, and ball milled at a speed of 200rpm for 16h. In order to compensate for the loss of lithium volatilization during high-temperature calcination, an excess of 10% of the lithium source is set. After the ball milling, the obtained mixture was dried in a blast oven at 60° C. for 24 hours to obtain a precursor powder. The obtained powder was transferred to a porcelain boat and calcined in a tube furnace at 900 °C for 12 h in an air atmosphere. The pre-sintered powder was ground again, and then calcined at 1200 °C in air for 24 h to obtain cubic Li 6.35 La 2.95 Rb 0.05 Zr 1.2 Ta 0.8 o 12 .
[0042] Weigh 1.12 g of polyethylene oxide and 0.48 g of polypropylene carbo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com