Co-expression cell of secretory anti-immune checkpoint antibody and tEGFR molecule and application thereof
An immune checkpoint, secreted technology, applied in the fields of transgenic cells, expression vectors, therapeutic compositions for cancer treatment, secreted antibodies, and lentivirus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
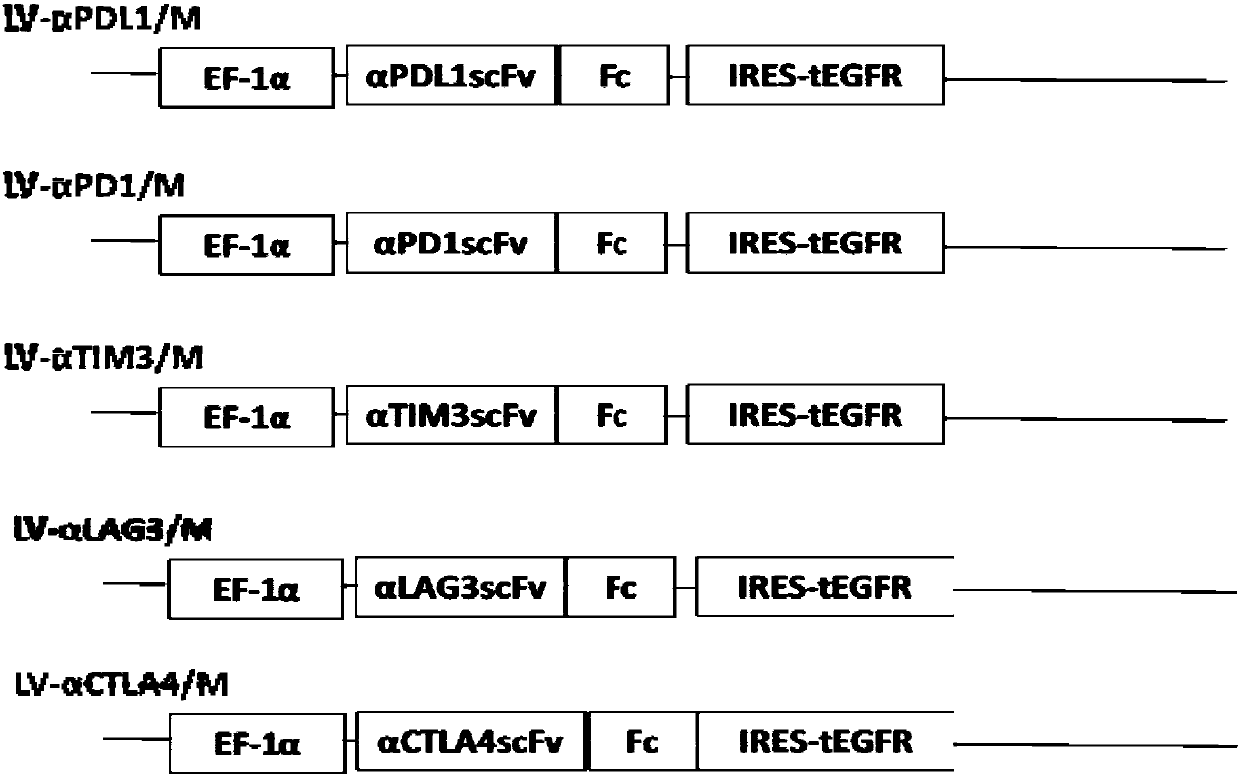
[0127] Example 1 Construction of vectors for co-expression of secreted anti-immune checkpoint antibodies and non-functional EGFR receptors
[0128] In this example, the inventors cloned the fusion gene encoding anti-human PD-L1 single-chain antibody and human IgG1 Fc into a lentiviral vector containing an EF-1 promoter. During the cloning process, the selected restriction enzymes are XbaI and NotI double enzyme digestion, and NotI and XhoI double enzyme digestion. Through enzyme digestion, ligation, screening and amplification of the target plasmid, the expression of secreted anti-immune checkpoint antibody is generated. Lentiviral plasmid (LV-αPDL1 scFv-Fc (LV-αPDL1). The inventors cloned the fusion gene encoding anti-human PD-1 single-chain antibody and human IgG4Fc into a lentiviral vector containing EF-1 promoter. During the cloning process, the selected restriction enzymes are XbaI and NotI double enzyme digestion, and NotI and XhoI double enzyme digestion. Through enzyme...
Embodiment 2
[0129] Example 2 PD-1 isolated from tumor patients + CD8 + T cells have killing activity against autologous tumor cells.
[0130] from HLA-A2 + PBLs (peripheral blood) and fresh tumor samples were obtained from patients with metastatic melanoma. PBLs were removed by venipuncture, subjected to gradient centrifugation (LSM; ICN Biomedicals Inc.), and stored frozen until analysis. Fresh tumor samples were minced under sterile conditions and digested with enzymes (the composition of the digestion solution was: RPMI-1640 medium containing L-glutamine [Lonza], 1mg / ml collagenase IV [Sigma-Aldrich ], 30U / ml DNase and antibiotics), the digestion conditions were overnight at room temperature or several hours at 37°C, and mechanical separation was performed intermittently using gentleMACS (Miltenyi Biotech). Tumor single-cell suspensions were used to obtain primary tumor cell lines and to isolate and amplify PD-1 + CD8 + T cells. PD-1 from tumor single cell suspension + CD8 ++ ...
Embodiment 3
[0132] Example 3 Lentiviral vector LV-αPDL1 / M transduction enhances PD-1 + CD8 + Tumor killing activity of T cells.
[0133] PD1 isolated from patients with metastatic melanoma + CD8 + T cells, PD1 - CD8 + T cells and unisolated CD8 + T cells were seeded on cell culture dishes lined with recombinant fibronectin fragments (FN ch–296; Retronectin) and transduced with lentiviruses, respectively LV-αPDL1 / M or empty LV-M transduction. Transduced T cells were expanded for cell killing assay. Melanoma target cells were used as target cells from the same patient. The target cells were treated with 100μCi (1Ci=37GBq) 51 Cr (PerkinElmer) labeled, after elution twice, matched effector T cells from the same patient were plated in a 96-well U-bottom culture dish at the specified effector / target ratio in the culture dish, and incubated at 37°C for 4 hours . 51 The amount of Cr released was calculated by gamma counting, and a dissolution rate was calculated for three samples. imag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com