Space charge transfer dendritic fluorescent material, preparation method thereof and organic light-emitting device
A dendritic and fluorescent technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problem of low device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0116] The present invention also provides a method for preparing a dendritic fluorescent polymer compound as described in any one of the above technical solutions, comprising the following steps:
[0117] 1) Under a protective atmosphere, the iodo compound X-1 is subjected to a Sonogashira coupling reaction with p-bromophenylacetylene to obtain an intermediate X-2;
[0118] 2) Under a protective atmosphere, the intermediate X-2 obtained in the above steps is subjected to a cyclotrimerization reaction to obtain intermediate X-3 and intermediate X-4 respectively;
[0119] 3) Under a protective atmosphere, after performing the first Buchwald–Hartwig reaction on the intermediate X-3 obtained in the above steps and the arylamine compound X-5, a dendritic fluorescent polymer compound with a specific structure of formula (I) is obtained;
[0120] Under a protective atmosphere, the intermediate X-4 obtained in the above steps and the arylamine compound X-5 are subjected to the second...
Embodiment 1
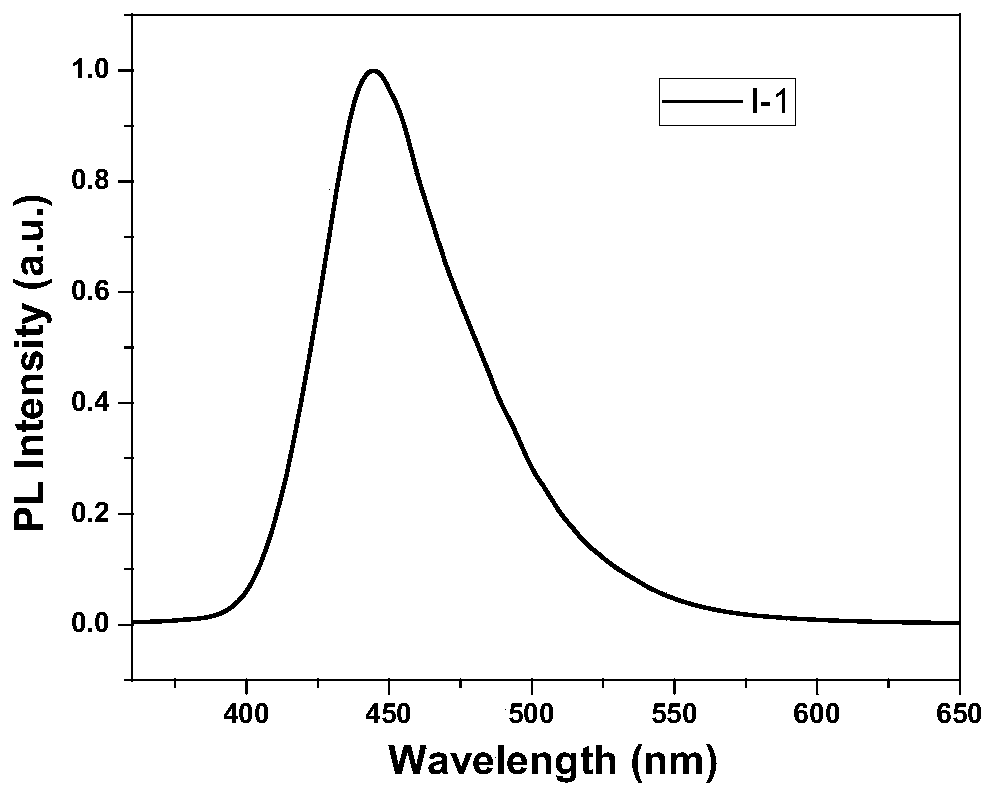
[0167] The chemical structure and synthetic route of I-1 are as follows:
[0168]
[0169] (1), the preparation of intermediate 3: add 1 (8.71g, 20mmol), 2 (4.35g, 24mmol), tetrakistriphenylphosphine palladium (1.16g, 1mmol) in the there-necked flask of 500 milliliters, iodide Copper (0.38g, 2mmol) and triphenylphosphine (0.53g, 2mmol) were replaced with argon and injected with 100ml of anhydrous and oxygen-free tetrahydrofuran and 100ml of triethylamine, respectively, and reacted at 60°C for 24 hours. Cool down to room temperature, add dilute hydrochloric acid and dichloromethane 100mL for extraction, wash twice with dilute hydrochloric acid solution, and wash several times with deionized water. The organic phase was separated, and 5.3 g of the compound with the structure shown in Intermediate 3 was obtained through column separation and desolvation, with a yield of 54%.
[0170] (2), the preparation of intermediate 4 and intermediate 5:
[0171] Under argon atmosphere, ...
Embodiment 2
[0175] The synthetic route is as follows:
[0176]
[0177] Under argon atmosphere, intermediate 5 (0.44g, 0.3mmol), 6 (0.51g, 1.8mmol), Pd 2 (dba) 3 (83mg, 0.09mmol), t-Bu 3 PHBF 4 (105mg, 0.36mmol), t-BuONa (0.35g, 3.6mmol), then inject 20mL of toluene, and react at 110°C for 24 hours. Cool down to room temperature, add deionized water and 100 mL of dichloromethane for extraction, and wash with deionized water several times. The organic phase was separated, and the fluorescent material II-10.29 g was obtained by column separation and solvent removal, with a yield of 47%. C147H126N12 elemental analysis (%): C, 85.68; H, 6.16; N, 8.16; MALDI-TOF (m / z): 2059
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com