Vaccine compound adjuvant system and its application in antigen
An adjuvant and vaccine technology, applied in the field of immunology, can solve the problem of no strengthening effect of cellular immunity, no strengthening of immune response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Stability experiment of a composite adjuvant system composed of double-stranded polynucleotide-ε-polylysine complex and aluminum adjuvant
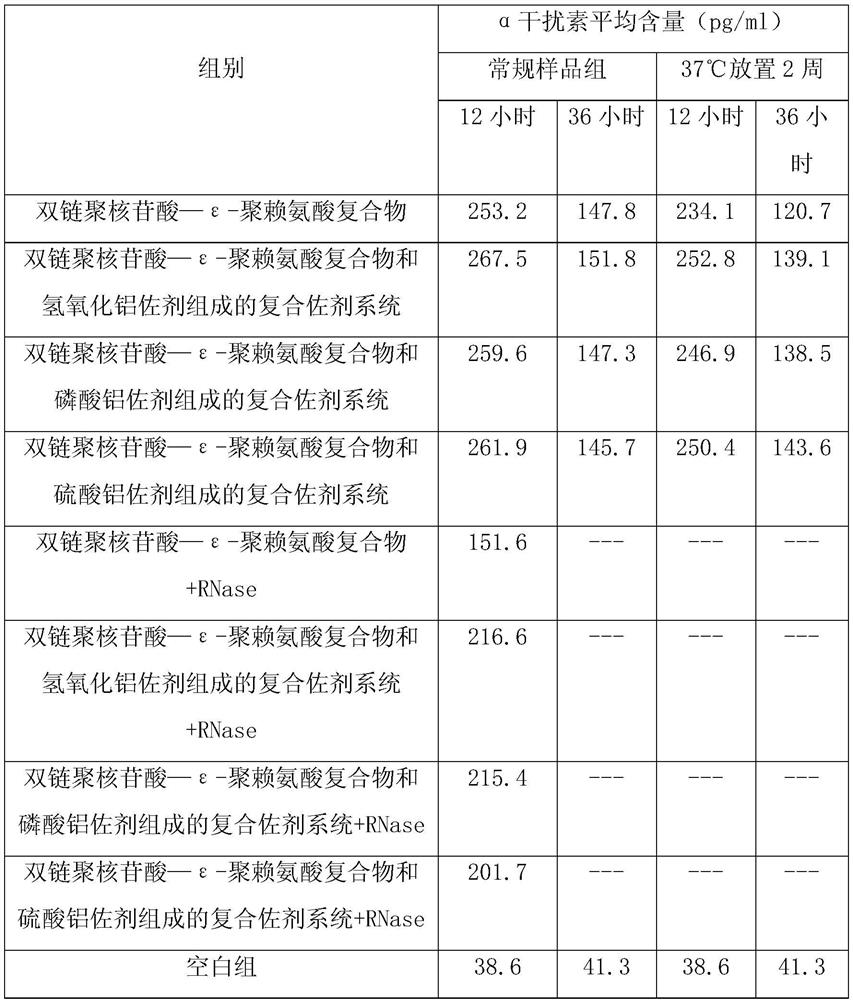
[0047] The 21-day-old chicks were randomly divided into eight groups, 10 in each group, and one group was injected with double-stranded polynucleotide-ε-polylysine complex (containing poly IC 2mg, ε-polylysine 1.0 mg, calcium chloride 0.4mmol / L), the second group was injected with a compound adjuvant system composed of double-stranded polynucleotide-ε-polylysine complex and aluminum adjuvant (each ml contains poly IC 2mg, ε- Polylysine 1.0mg, calcium chloride 0.4mmol / L, aluminum hydroxide adjuvant 1.6mg), the third group was injected with double-stranded polynucleotide-ε-polylysine complex and aluminum adjuvant Adjuvant system (each ml contains poly IC 2mg, ε-polylysine 1.0mg, calcium chloride 0.4mmol / L, aluminum phosphate adjuvant 1.0mg), the fourth group was injected with double-stranded polynucleotide—ε-polylysine Com...
Embodiment 2
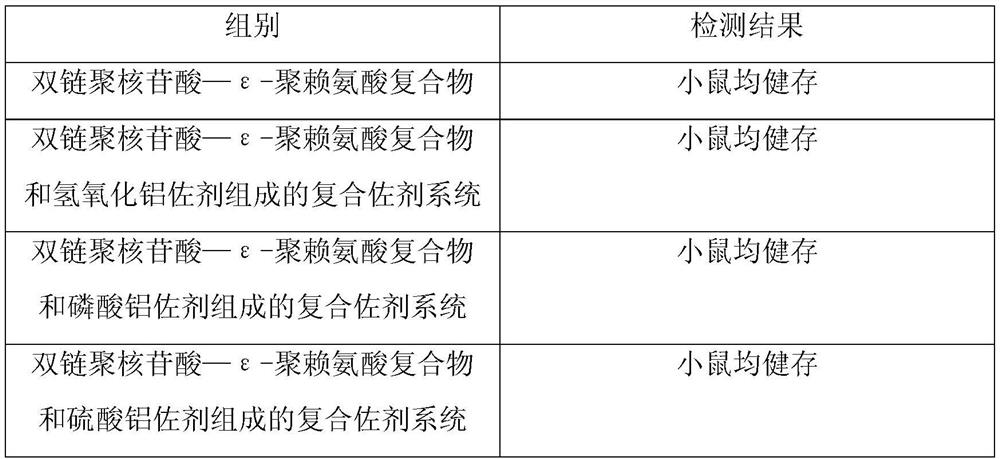
[0050] Example 2: Detection of abnormal toxicity of the compound adjuvant system composed of double-stranded polynucleotide-ε-polylysine complex and aluminum adjuvant
[0051] The compound adjuvant system composed of double-stranded polynucleotide-ε-polylysine complex and aluminum adjuvant prepared in Example 1 was tested for abnormal toxicity according to the method of "Chinese Pharmacopoeia", and the test results are as follows:
[0052]
[0053] The test results show that the compound adjuvant system composed of the double-stranded polynucleotide-ε-polylysine complex prepared in Example 1 and aluminum adjuvant is qualified for abnormal toxicity test in mice.
Embodiment 3
[0054] Example 3: Potency detection of the compound adjuvant system composed of double-stranded polynucleotide-ε-polylysine complex and aluminum adjuvant combined with inactivated rabies vaccine
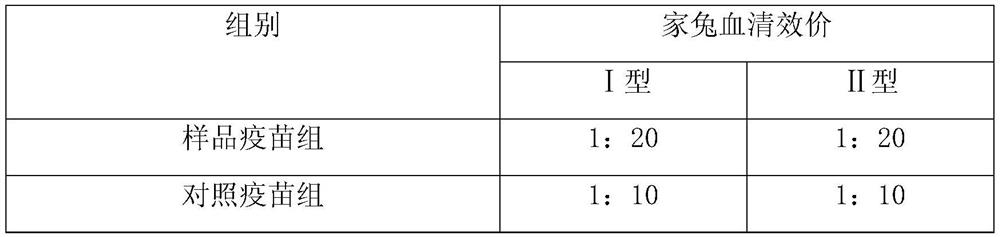
[0055] The composite adjuvant system that the double-stranded polynucleotide-ε-polylysine complex prepared in Example 1 and aluminum phosphate adjuvant (aluminum phosphate adjuvant content 1.0mg / ml) is formed and inactivated rabies virus antigen Fully mixed at a ratio of 1:1, as the sample vaccine group, and the inactivated rabies virus antigen as the control vaccine group. Then carry out potency detection by rabies vaccine titer assay method (NIH method), and test result is as follows:
[0056] group Vaccine potency (IU / ml) Sample Vaccine Group 6.6 control vaccine group 4.0
[0057] The test results showed that the potency of the sample vaccine group was higher than that of the control vaccine group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com